Abstract
Background
With a technical improvement of the assay system, automated immunoassay analyzers for hepatitis B surface antibody (anti-HBs) are widely used. However, some discrepancies between assays are still being reported. We compared the qualitative and quantitative results of three kinds of anti-HBs assays.
Methods
Serum samples were collected from 517 patients and anti-HBs were determined using AxSYM AUSAB, Bayer ADVIA Centaur, and Roche Elecsys assay systems.
Results
The concordance rates between the three assays were 95.1% (543/571). The concordance rates were 97.7% between Centaur and Elecsys, 96.3% between AxSYM and Centaur, and 95.6% between AxSYM and Elecsys. Their correlation coefficients for quantitative results were 0.97, 0.94, and 0.93 in the same order. Twenty-eight specimens showed discrepant results, and all of them had antibody values below 31.5 mIU/mL.
References
1. Cha YJ, Kwon SY, Kum DG, Kim SW, Kim TY, Kim JR, et al. Annual report on external quality assessment in immunoserology in Korea (2003). J Lab Med Qual Assur. 2004; 26:47–69.
2. Davidson M, Krugman S. Recombinant yeast hepatitis B vaccine compared with plasma-derived vaccine: immunogenicity and effect of a booster dose. J Infect. 1986; 13:S31–8.

3. Player VA, White D. Comparison of an ELISA system for the quantification of hepatitis B antibody with an automated and a semiautomated system. J Virol Methods. 1993; 45:67–72.

4. Whang DH, Shin BM. Comparison of four-assay system for the quantification of hepatitis B surface antibody. Korean J Lab Med. 2002; 22:424–30.
5. Heijtink RA, Schneeberger PM, Postma B, Crombach W. Anti-HBs levels after hepatitis B immunisation depend on test reagents: routinely determined 10 and 100 IU/l seroprotection levels unreliable. Vaccine. 2002; 20:2899–905.

6. McCartney RA, Harbour J, Roome AP, Caul EO. Comparison of enhanced chemiluminescence and microparticle enzyme immunoassay for the measurement of hepatitis B surface antibody. Vaccine. 1993; 11:941–5.

7. Whang DH, Um TH. Comparison of immunochromatography assays and quantitative immunoassays for detecting HBsAg and anti-HBs. Korean J Lab Med. 2005; 25:186–91.
8. Oh JH, Kim TY, Yoon HJ, Min HS, Lee HR, Choi TY. Evaluation of Genedia HBsAg Rapid Genedia Anti-HBs Rapid for the screening of HBsAg and Anti-HBs. Korean J Clin Pathol. 1999; 19:114–7.
9. Taylor P, Pickard G, Gammie A, Atkins M. Comparison of the AD-VIA Centaur and Abbott AxSYM immunoassay systems for a routine diagnostic virology laboratory. J Clin Virol. 2004; 30:S11–5.

10. Ostrow DH, Edwards B, Kimes D, Macioszek J, Irace H, Nelson L, et al. Quantitation of hepatitis B surface antibody by an automated microparticle enzyme immunoassay. J Virol Methods. 1991; 32:265–76.

11. Doche C, Thome M, Dimet I, Bienvenu J. Evaluation of the fully automated Cobas Core enzyme immunoassay for the quantitation of antibodies against hepatitis B virus surface antigen. Eur J Clin Chem Clin Biochem. 1996; 34:365–8.
Fig. 1.
Regression graphs for anti-HBs (mIU/mL) excluding the results above 1,000 mIU/mL. (A) Elecsys vs Centaur, (B) AxSYM vs Elecsys, (C) AxSYM vs Centaur.
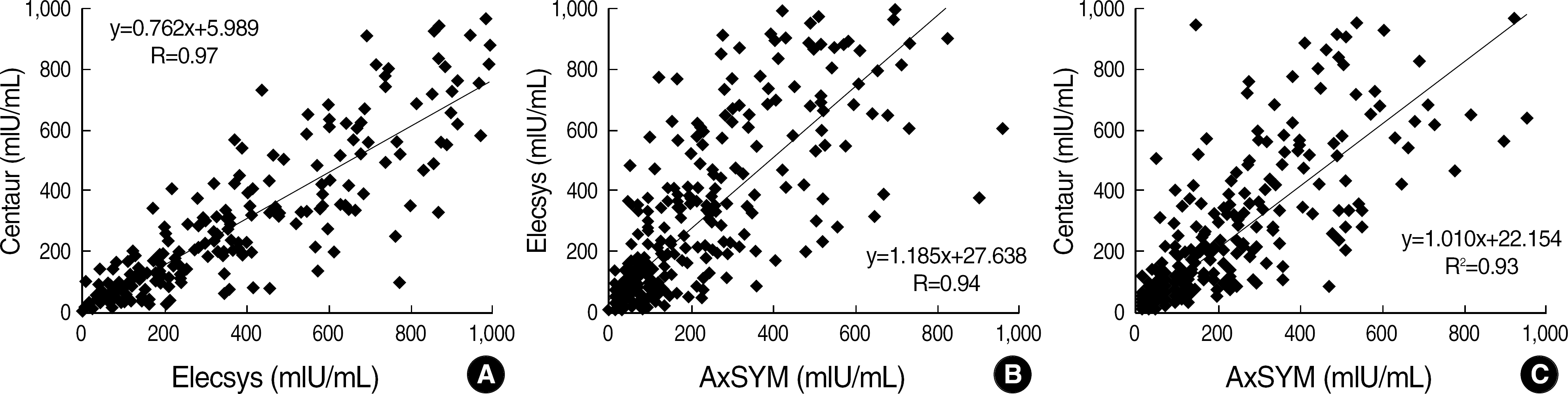
Table 1.
Comparison of the results between the three anti-HBs assay kits (n=571)
|
Results |
No. of specimens (%) | ||
|---|---|---|---|
| Elecsys | Centaur | AxSYM | |
| P | P | P | 427 (74.8%) |
| P | P | N | 10 (1.8%) |
| N | P | P | 6 (1.1%) |
| P | N | P | 1 (0.2%) |
| N | N | P | 5 (0.9%) |
| P | N | N | 4 (0.7%) |
| N | P | N | 2 (0.4%) |
| N | N | N | 116 (20.3%) |
Table 2.
Specimens showing discrepant results between the three assay kit (mIU/mL)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download