Abstract
Background
The CD34+ cell dose and infused number of committed progenitor cells in transplantation are important factors in hematologic engraftment. However, the relationship between expansion potential of progenitor cells and hematologic engraftment remains controversial. We evaluated whether expansion potential of progenitor cells is a predictive factor of post-transplantation hematologic engraftment.
Methods
Mononuclear cells isolated from mobilized peripheral blood and bone marrow were cultured with cytokine cocktail for 7 days. Progenitor cells and committed progenitors were analyzed using stem cell markers (CD34 and CD133) and lineage specific markers. Hematologic engraftment was defined as neutrophil counts over 500/μL and platelet counts over 20,000/μL without transfusion. Acute and chronic graft-versus-host disease (GVHD) were investigated.
Results
There was inverse tendency between the number and fold expansion of progenitor cells or committed (granulocytic or megakaryocytic) progenitors and time to engraftment. Especially, fold expansion of CD34+/CD33+ cells was significantly correlated with time to neutrophil engraftment in bone marrow transplantation (r=-0.56, P=0.04). The infused number and fold expansion of lymphoid progenitors were not related to the occurrence of acute or chronic GVHD.
Conclusions
We could not prove that expansion potential of progenitor cells and committed progenitor cells is correlated to hematologic engraftment although there is a correlation between CD34+/CD33+ cells and time to neutrophil engraftment. But, a further study on the value of expansion potential is required because there is an inverse tendency.
References
1. Krause DS, Fackler MJ, Civin CI, May WS. CD34: structure, biology, and clinical utility. Blood. 1996; 87:1–13.
2. Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997; 90:5002–12.

3. Siena S, Bregni M, Brando B, Belli N, Ravagnani F, Gandola L, et al. Flow cytometry for clinical estimation of circulating hematopoietic progenitors for autologous transplantation in cancer patients. Blood. 1991; 77:400–9.

4. Faucher C, Le Corroller AG, Chabannon C, Viens P, Stoppa AM, Bouabdallah R, et al. Autologous transplantation of blood stem cells mobilized with filgrastim alone in 93 patients with malignancies: the number of CD34+ cells reinfused is the only factor predicting both granulocyte and platelet recovery. J Hematother. 1996; 5:663–70.
5. McAdams TA, Winter JN, Miller WM, Papoutsakis ET. Hematopoietic cell culture therapies (Part II): Clinical aspects and applications. Trends Biotechnol. 1996; 14:388–96.
6. Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collection in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995; 10:3961–9.
7. Zubair A, Zahrieh D, Daley H, Schott D, Gribben JG, Freedman A, et al. Early neutrophil engraftment following autologous BMT provides a functional predictor of long-term hematopoietic reconstitution. Transfusion. 2003; 43:614–21.

8. Hogge DE, Lambie K, Sutherland HJ, Benny WB, Dalal B, Currie C, et al. Quantitation of primitive and lineage-committed progenitors in mobilized peripheral blood for prediction of platelet recovery post autologous transplant. Bone Marrow Transplant. 2000; 25:589–98.

9. Astori G, Malangone W, Adami V, Risso A, Dorotea L, Falasca E, et al. A novel protocol that allows short-term stem cell expansion of both committed and pluripotent hematopoietic progenitor cells suitable for clinical use. Blood Cells Mol Dis. 2001; 27:715–24.

10. Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996; 5:213–26.
11. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995; 15:825–8.
12. Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980; 69:204–17.
13. Przepiorka D, Anderlini P, Saliba R, Cleary K, Mehra R, Khouri I, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 2001; 98:1695–700.

14. Wang B, Kang ZZ, Chi ZY, Xu L, Tan WS. Study on the ex vivo expansion characteristics of umbilical cord blood CD34+ cells and mononuclear cells. Zhonghua Xue Ye Xue Za Zhi. 2003; 24:74–7.
15. De Bruyn C, Delforge A, Bron D, Bernier M, Massy M, De Hemptinne D, et al. Ex vivo expansion of CD34+CD38-cord blood cells. J Hematother. 1997; 6:93–102.
16. Koller MR, Manchel I, Brott DA, Palsson BO. Donor-to-donor variability in the expansion potential of human bone marrow cells is reduced by accessory cells but not only soluble growth factors. Exp Hematol. 1996; 24:1484–93.
17. Ketterer N, Salles G, Raba M, Espinouse D, Sonet A, Tremisi P, et al. High CD34(+) cell counts decrease hematologic toxicity of autologous peripheral blood progenitor cell transplantation. Blood. 1998; 91:3148–55.

18. To LB, Roberts MM, Haylock DN, Dyson PG, Branford AL, Thorp D, et al. Comparison of haematological recovery times and supportive care requirements of autologous recovery phase peripheral blood stem cell transplants, autologous bone marrow transplants and allogeneic bone marrow transplants. Bone Marrow Transplant. 1992; 9:277–84.
19. Dercksen MW, Rodenhuis S, Dirkson MK, Schaasberg WP, Baars JW, van der Wall E, et al. Subsets of CD34+ cells and rapid hematopoietic recovery after peripheral-blood stem-cell transplantation. J Clin Oncol. 1995; 13:1922–32.

20. Feng R, Shimazaki C, Inaba T, Takahashi R, Hirai H, Kikuta T, et al. CD34+/CD41a+ cells best predict platelet recovery after autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1998; 21:1217–22.

21. Drayer AL, Smit Sibinga CT, Esselink MT, de Wolf JT, Vellenga E. In vitro megakaryocyte expansion in patients with delayed platelet engraftment after autologous stem cell transplantation. Ann Hematol. 2002; 81:192–7.
22. Watanabe T, Dave B, Heimann DG, Jackson JD, Kessinger A, Talmadge JE. Cell adhesion molecule expression on CD34+ cells in grafts and time to myeloid and platelet recovery after autologous stem cell transplantation. Exp Hematol. 1998; 26:10–8.
23. Peled A, Kollet O, Ponomaryov T, Petit I, Franitza S, Grabovsky V, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000; 95:3289–96.

24. Keever-Taylor CA, Bredeson C, Loberiza FR, Casper JT, Lawton C, Rizzo D, et al. Analysis of risk factor for the development of GVHD after T cell-depleted allogeneic BMT: effect of HLA disparity, ABO incompatibility, and method of T-cell depletion. Biol Blood Marrow Transplant. 2001; 7:620–30.
Fig. 1.
Three-color flow cytogram for the measurement of hematopoietic progenitor cells. (A) Measurement of CD45+, CD34+ or CD133+(AC133+) cells. (B) Measurement of CD45+, CD34+ and CD19+ cells.
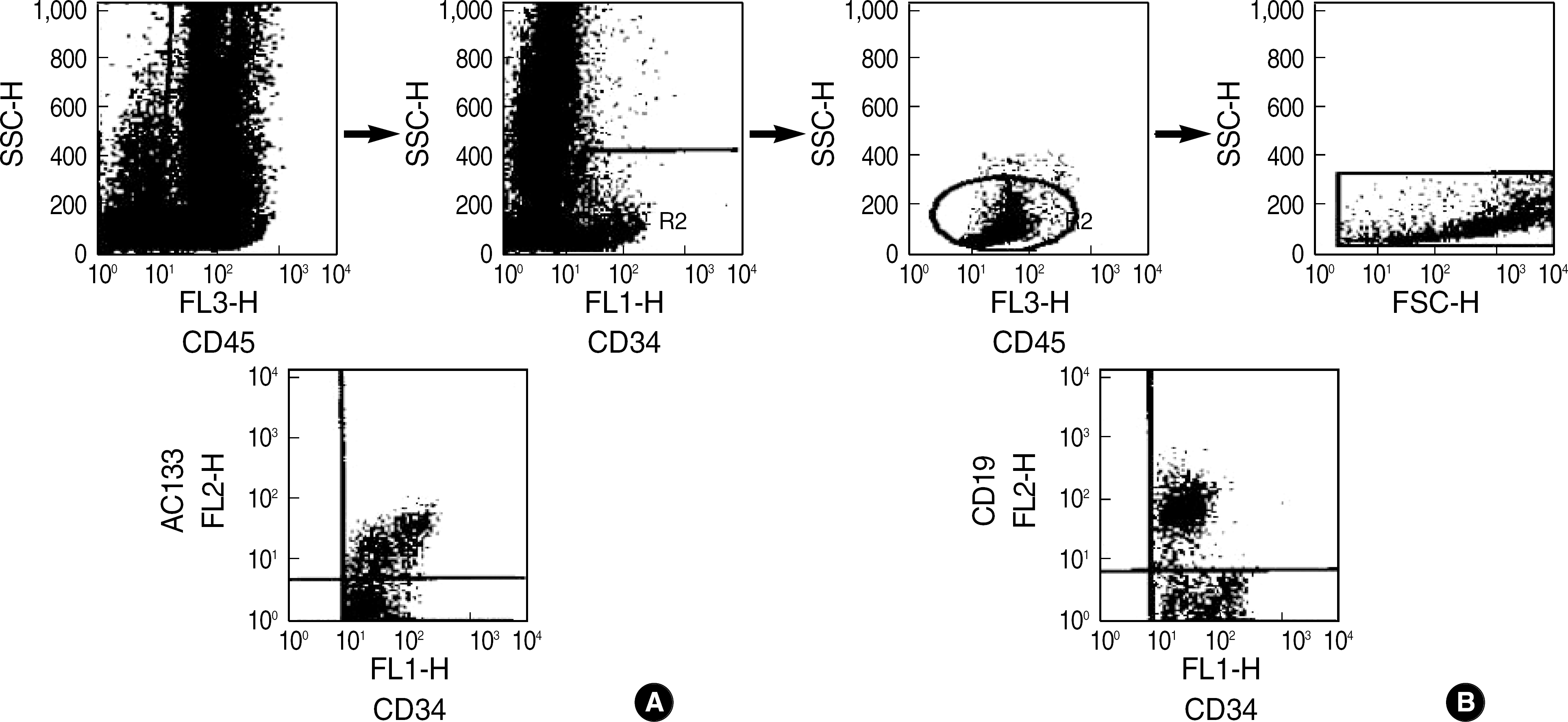
Fig. 2.
Correlation of expansion potential of progenitor cells with neutrophil or platelet recovery (days) after bone marrow transplantation (n=14).
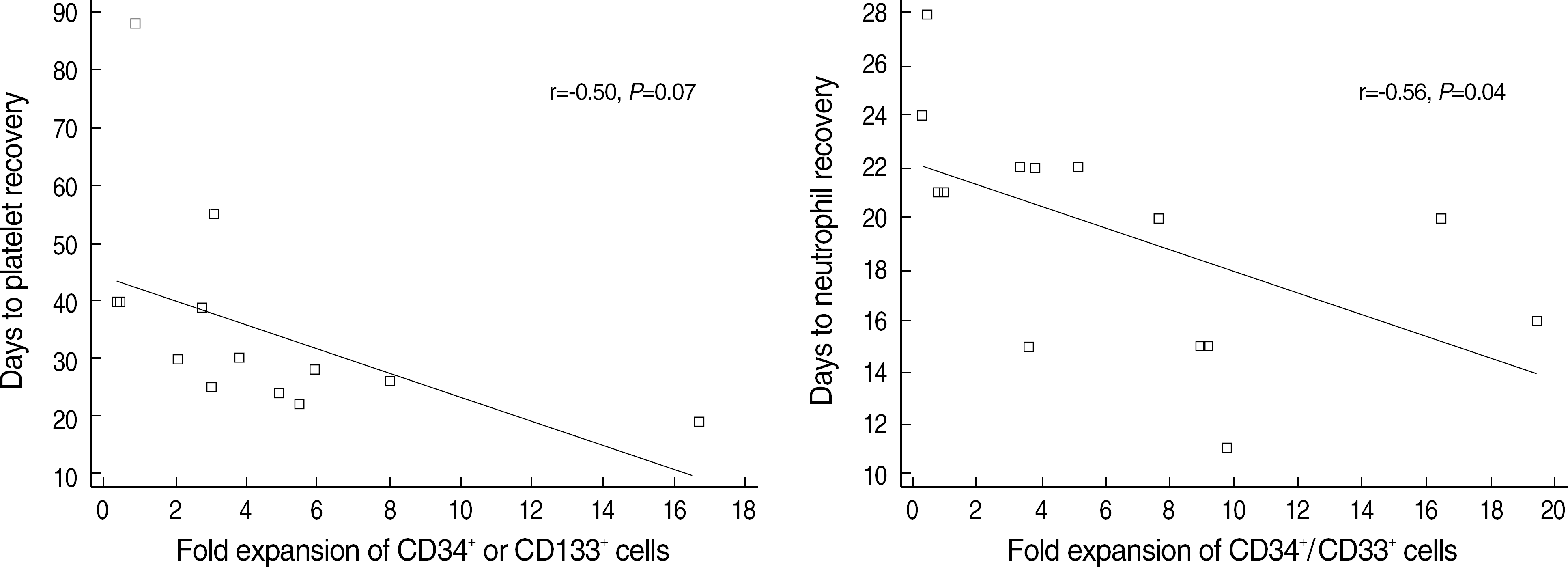
Table 1.
Number and fold expansion of mononuclear cells and progenitor cells before and after 7-day culture
Table 2.
Expansion potential of progenitor cells in mPB and BM
Table 3.
Correlation of infused number (×106/kg) or expansion potential of lymphoid progenitor cells with the occurrence of acute GVHD
Table 4.
Correlation of infused number (×106/kg) or expansion potential of lymphoid progenitor cells with the occurrence of acute GVHD




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download