Abstract
Background
Human telomerase is a ribonucleoprotein polymerase, which synthesizes telomeric repeat sequences, and human telomerase reverse transcriptase (hTERT) has been identified as the catalytic subunit, as well as the rate-limiting component, of telomerase. In this study, we attempted to identify the modulators of telomerase, and to determine the molecular mechanisms underlying cis-platin-induced apoptosis.
Methods
To determine the role of telomerase in cisplatin-induced apoptosis, we measured telomerase activity and analyzed apoptosis using PI and trypan blue staining. Also, we inhibited the caspase activations using Z-VAD-fmk to analyze the effects on expression of hTERT protein. Finally, we induced the transient co-expression of the Bcl-2 and Bak genes in HEK293 cells, and then, the telomerase activity and expression of hTERT were evaluated.
Results
In the Bcl-2-overexpressing HeLa cells, telomerase activity was more enhanced, and cell death was reduced to 40–50% that of the mock controls. This finding suggests that Bcl-2-induced telomerase activity exerts an antiapoptotic effect in cisplatin-induced death. As caspase activation was inhibited via Z-VAD-fmk, the hTERT protein was recovered in the mock controls, but not in the Bcl-2-overexpressing cells. This suggests that the expression of hTERT can be regulated by caspases, but Bcl-2 was located within the upstream pathway. Moreover, when the Bcl-2 and Bak genes were co-transfected into the HEK293, both telomerase activity and hTERT protein were prominently reduced.
Go to : 
References
3. Stewart SA, Hahn WC, O'Connor BF, Banner EN, Lundberg AS, Modha P, et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc Natl Acad Sci USA. 2002; 99:12606–11.

4. Kondo Y, Kondo S, Tanaka Y, Haqqi T, Barna BP, Cowell JK. Inhibition of telomerase increases the susceptibility of human malignant glioblastoma cells to cisplatin-induced apoptosis. Oncogene. 1998; 16:2243–8.

5. Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999; 274:7264–71.

6. Fu W, Killen M, Culmsee C, Dhar S, Pandita TK, Mattson MP. The catalytic subunit of telomerase is expressed in developing brain neurons and serves a cell survival-promoting function. J Mol Neurosci. 2000; 14:3–15.

7. Kushner DM, Paranjape JM, Bandyopadhyay B, Cramer H, Leaman DW, Kennedy AW, et al. 2–5A antisense directed against telomerase RNA produces apoptosis in ovarian cancer cells. Gynecol Oncol. 2000; 76:183–92.

8. Dudognon C, Pendino F, Hillion J, Saumet A, Lanotte M, Segal-Bendirdjian E. Death receptor signaling regulatory function for telomerase: hTERT abolishes TRAIL-induced apoptosis, independently of telomere maintenance. Oncogene. 2004; 23:7469–74.

9. Mattson MP, Klapper W. Emerging roles for telomerase in neuronal development and apoptosis. J Neurosci Res. 2001; 63:1–9.

10. Hemann MT, Rudolph KL, Strong MA, DePinho RA, Chin L, Greider CW. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol Biol Cell. 2001; 12:2023–30.

11. Ichiyoshi H, Kiyozuka Y, Kishimoto Y, Fukuhara S, Tsubura A. Massive telomere loss and telomerase RNA expression in dexamethasone-induced apoptosis in mouse thymocytes. Exp Mol Pathol. 2003; 75:178–86.

12. Zhang P, Chan SL, Fu W, Mendoza M, Mattson MP. TERT suppresses apoptotis at a premitochondrial step by a mechanism requiring reverse transcriptase activity and 14–3–3 protein-binding ability. FASEB J. 2003; 17:767–9.
13. Gorbunova V, Seluanov A, Pereira-Smith OM. Expression of human telomerase (hTERT) does not prevent stress-induced senescence in normal human fibroblasts but protects the cells from stress-induced apoptosis and necrosis. J Biol Chem. 2002; 277:38540–9.

14. Holt SE, Glinsky VV, Ivanova AB, Glinsky GV. Resistance to apoptosis in human cells conferred by telomerase function and telomere stability. Mol Carcinogen. 1999; 25:241–8.

15. Mandal M, Kumar R. Bcl-2 modulates telomerase activity. J Biol Chem. 1997; 272:14183–7.
16. MacNamara B, Wang W, Chen Z, Hou M, Mazur J, Gruber A, et al. Telomerase activity in relation to pro- and anti-apoptotic protein expression in high grade non-Hodgkin's lymphomas. Haematologica. 2001; 86:386–93.
17. Loehrer PJ, Einhorn LH. Drugs five years later. Cisplatin. Ann Intern Med. 1984; 100:704–13.
18. Chu G, Mantin R, Shen YM, Baskett G, Sussman H. Massive cisplatin overdose by accidental substitution for carboplatin. Toxicity and management. Cancer. 1993; 72:3707–14.

19. Folini M, Brambilla C, Villa R, Gandellini P, Vignati S, Paduano F, et al. Antisense oligonucleotide-mediated inhibition of hTERT, but not hTERC, induces rapid cell growth decline and apoptosis in the absence of telomere shortening in human prostate cancer cells. Eur J Cancer. 2005; 41:624–34.

20. Kraemer K, Fuessel S, Schmidt U, Kotzsch M, Schwenzer B, Wirth MP, et al. Antisense-mediated hTERT inhibition specifically reduces the growth of human bladder cancer cells. Clin Cancer Res. 2003; 9:3794–800.
21. Tahara H, Shin-Ya K, Seimiya H, Yamada H, Tsuruo T, Ide T. G-Quadruplex stabilization by telomestatin induces TRF2 protein dissociation from telomeres and anaphase bridge formation accompanied by loss of the 3′ telomeric overhang in cancer cells. Oncogene. 2006; 25:1955–66.

22. Tauchi T, Shin-Ya K, Sashida G, Sumi M, Okabe S, Ohyashiki JH, et al. Telomerase inhibition with a novel G-quadruplex-interactive agent, telomestatin: in vitro and in vivo studies in acute leukemia. Oncogene. 2006. in press.

23. Pallini R, Sorrentino A, Pierconti F, Maggiano N, Faggi R, Montano N, et al. Telomerase inhibition by stable RNA interference impairs tumor growth and angiogenesis in glioblastoma xenografts. Int J Cancer. 2006; 118:2158–67.

24. Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994; 369:321–3.
Go to : 
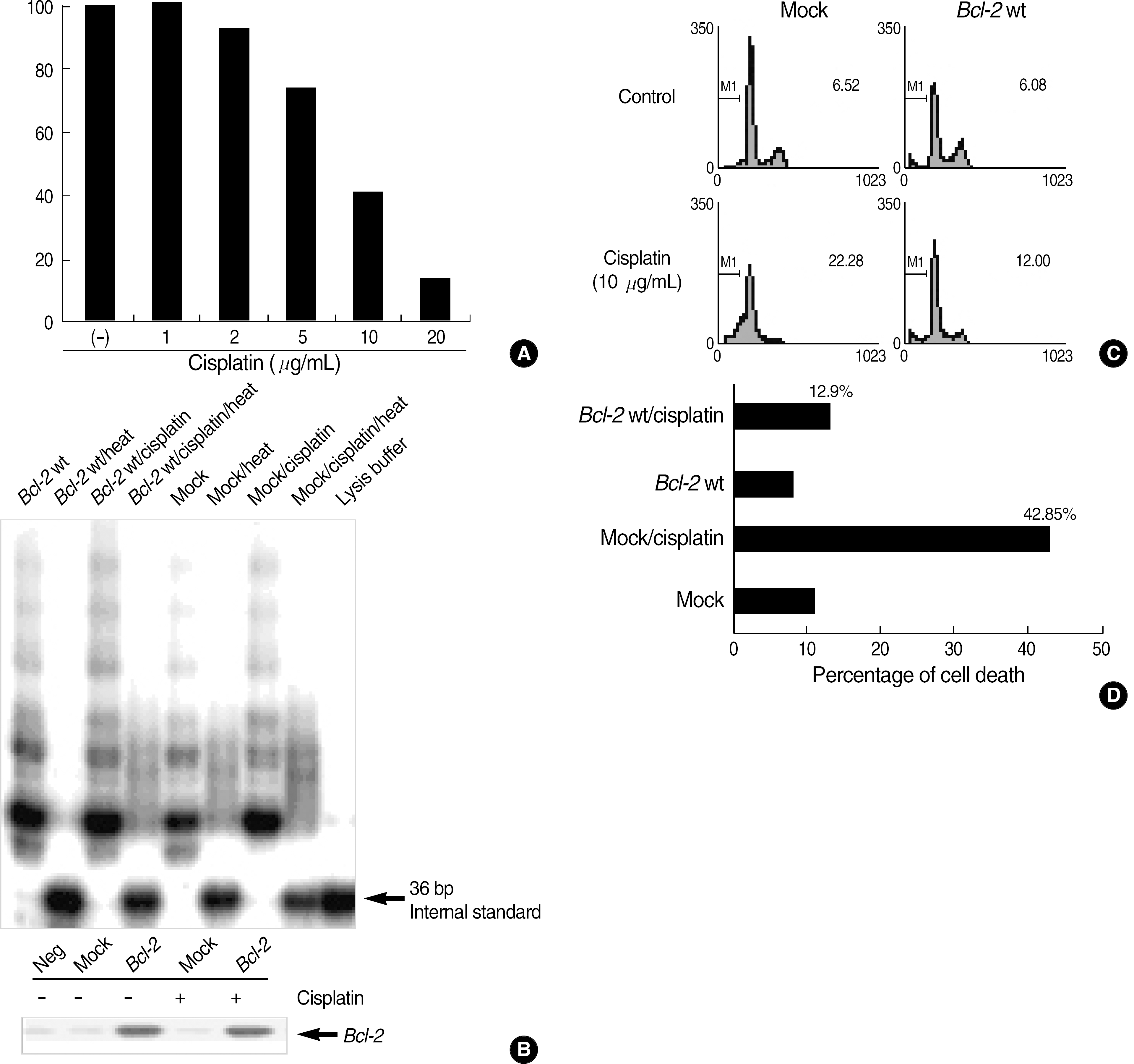 | Fig. 1.Telomerase activity in cisplatin-induced cell death. (A) Viability of HeLa cells were decreased by cisplatin dose-dependently. The listed values of optical density (OD) are expressed as the means ±SD of triplicates. (B) Bcl-2 wt expression enhanced the telomerase activity contrast to mock controls. Heat-inactivated extract was used as a control for specificity. (C, D) Fifty percents of apoptotic cells by cisplatin were reduced by Bcl-2 wt expression. Apoptotic cell death was evaluated via propidium iodide staining (C) and trypan blue staining (D). |
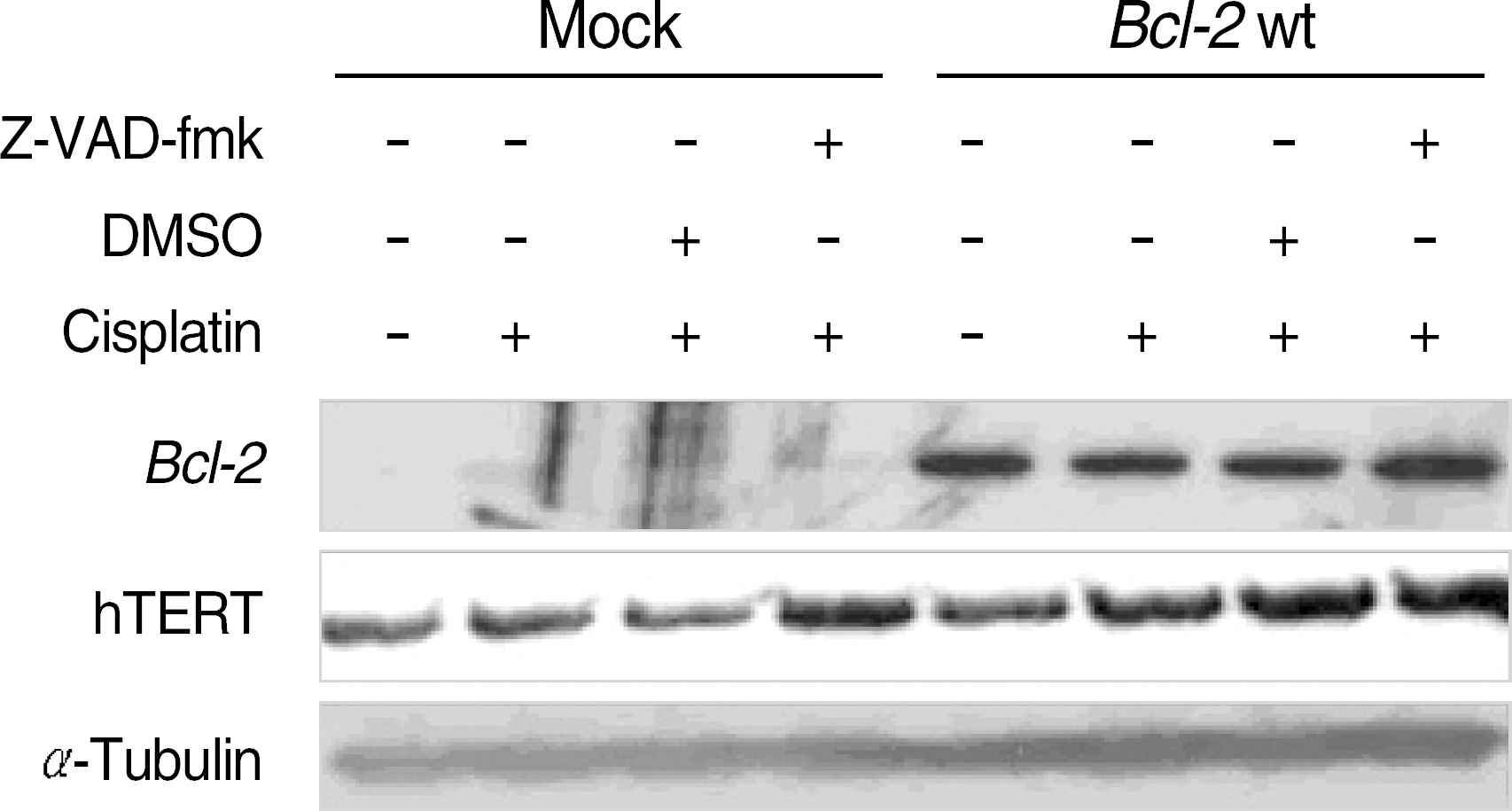 | Fig. 2.hTERT expression by Z-VAD-fmk inhibition in cisplatin-induced cell death. Bcl-2 and hTERT expression were evaluated via Western blot analysis. hTERT expression was changed by caspase inhibition. hTERT protein level was more increased by pretreatment of Z-VAD-fmk in mock control cells but not in Bcl-2 wt expressing cells. |
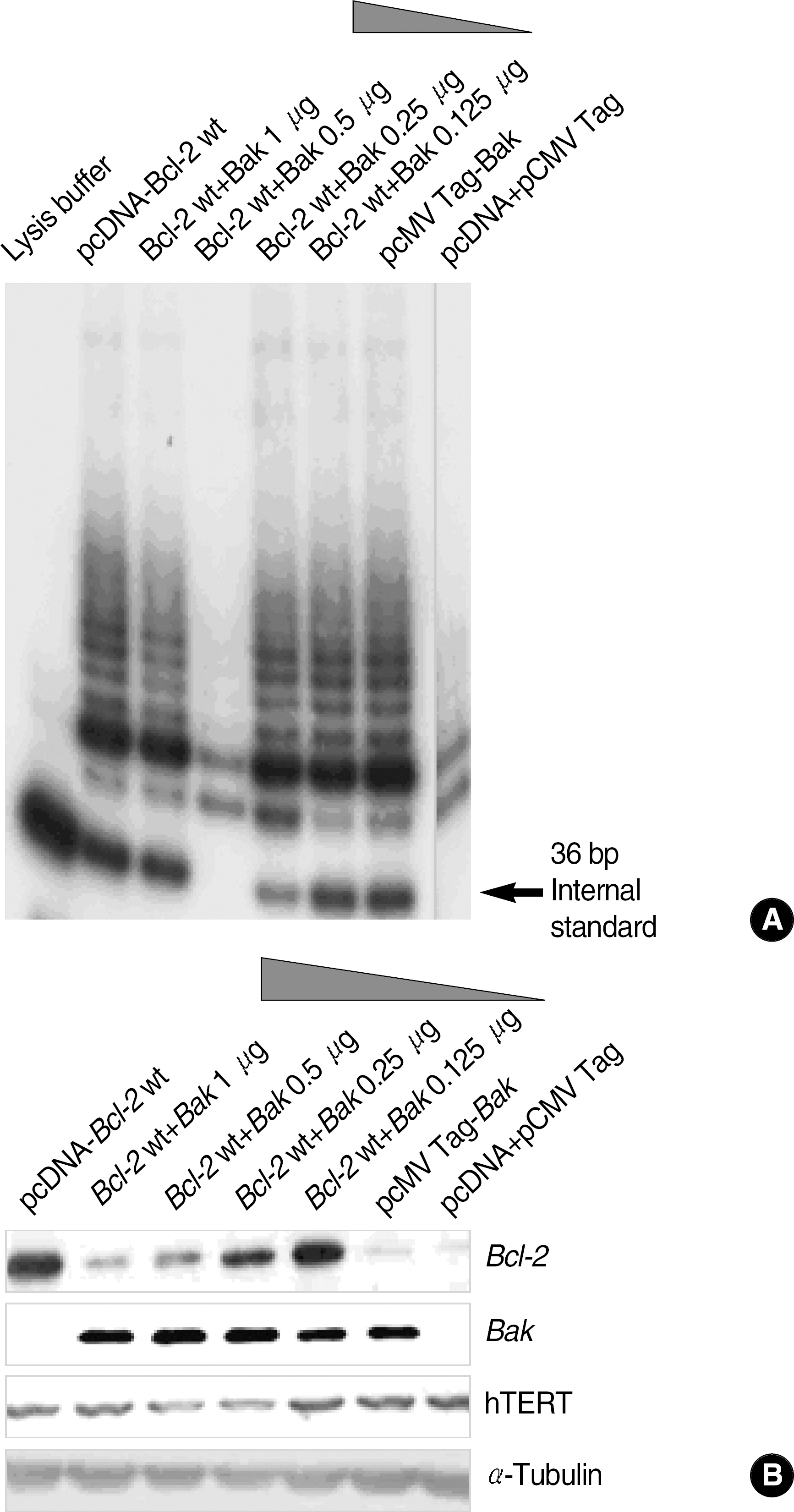 | Fig. 3.Telomerase activity and hTERT expression by interaction of Bcl-2with Bak. (A) HEK293 cells were co-transfected with a Bcl-2 wt (0.5 μg) and Bak (1–0.125 μg), or empty vectors. After 24 hr of incubation, telomerase activity was measured. Telomerase activity in Bcl-2and 0.5 μg of Bak co-expressing cells was reduced in contrast to other Bcl-2wt expressing cells. (B) Western blot analysis for the detection of Bcl-2and Bak or hTERT. Reduced expression of hTERT appeared in Bcl-2 wt and 0.25–0.5 μg of Bak co-expressing cells. Alpha tubulin was utilized to ensure equal loading.
*This study was supported by grants of FG05–40–01, NTM 0020213 of the 21C frontier function human genome project from ministry of science & technology of Korea.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download