Abstract
Background
Hepatitis B surface antigen (HBsAg) is one of the most important serological markers used to diagnose hepatitis B virus (HBV) infection. Automated immunoassays have been developed, meeting the current clinical requirement of HBsAg assays over the years. This study was performed to determine the degree of agreements between 3 kinds of HBsAg assay systems.
Methods
Serum samples from 425 patients were assayed by the HBsAg assay systems of Elecsys (Roche Diagnostics, Germany), ADVIA Centaur (Bayer Diagnostics, USA), and AxSYM (Abbott Laboratories, USA).
Results
The concordance rates among the 3 assays were 100%. A total of 249 (58.6%) specimens were positive, and their index values showed a weak correlation between the 3 assays; nevertheless, positive specimens with low levels (<10) of index values in one system also presented low values in other systems, and all of them were confirmed by neutralization assays.
References
1. Song SM, Oh WI, Kim DW. Evaluation of serologic marker tests for hepatitis B viral infection using the automated immunoassay system ARCHITECT i2000. Korean J Clin Pathol. 2002; 22:42–6.
3. Stevens CE, Taylor PE, Tong MJ, Toy PT, Vyas GN, Nair PV, et al. Yeast-recombinant hepatitis B vaccine. Efficacy with hepatitis B immune globulin in prevention of perinatal hepatitis B virus transmission. JAMA. 1987; 257:2612–6.

4. Moerman B, Moons V, Sommer H, Schmitt Y, Stetter M. Evaluation of sensitivity for wild type and mutant forms of hepatitis B surface antigen by four commercial HBsAg assays. Clin Lab. 2004; 50:159–62.
5. Taylor P, Pickard G, Gammie A, Atkins M. Comparison of the ADVIA Centaur and Abbott AxSYM immunoassay systems for a routine diagnostic virology laboratory. J Clin Virol. 2004; 30:S11–5.

6. Chen D, Kaplan L, Liu Q. Evaluation of two chemiluminescent immunoassays of ADVIA Centaur for hepatitis B serology markers. Clin Chim Acta. 2005; 355:41–5.

7. Weber B, Dengler T, Berger A, Doerr HW, Rabenau H. Evaluation of two new automated assays for hepatitis B virus surface antigen (HBsAg) detection: IMMULITE HBsAg and IMMULITE 2000 HBsAg. J Clin Microbiol. 2003; 41:135–43.

8. Bonino F, Hoyer B, Nelson J, Engle R, Verme G, Gerin J. Hepatitis B virus DNA in the sera of HBsAg carriers: a marker of active hepatitis B virus replication in the liver. Hepatology. 1981; 1:386–91.

9. van Helden J, Denoyel GA. Experience with the IVDD performance evaluations of the ADVIA Centaur infectious disease assays. J Clin Virol. 2004; 30:S16–8.

10. Song BC, Kim SH, Kim H, Ying YH, Kim HJ, Kim YJ, et al. Prevalence of naturally occurring surface antigen variants of hepatitis B virus in Korean patients infected chronically. J Med Virol. 2005; 76:194–202.

11. Park JW, Yoon JH, Hwang YJ, Lee HS, Kim CY. Mutations at the gene encoding the ‘a’ determinant of HBsAg in chronic hepatitis B patients with concurrent HBsAg and anti-HBs positivity. Korean J Gastroenterol. 1997; 29:182–91.
12. Cha YJ. Detection of hepatitis B virus surface antigen mutants. Korean J Lab Med. 2005; 25:442–7.
Fig. 1.
Index values of negative samples for HBsAg assays. Elecsys and Centaur use a cut off <1.0 and AxSYM use <2.0 for negatives. Plot indicates the median, 10th, 25th, 75th, and 90th percentiles as lines, vertical boxes and error bars.
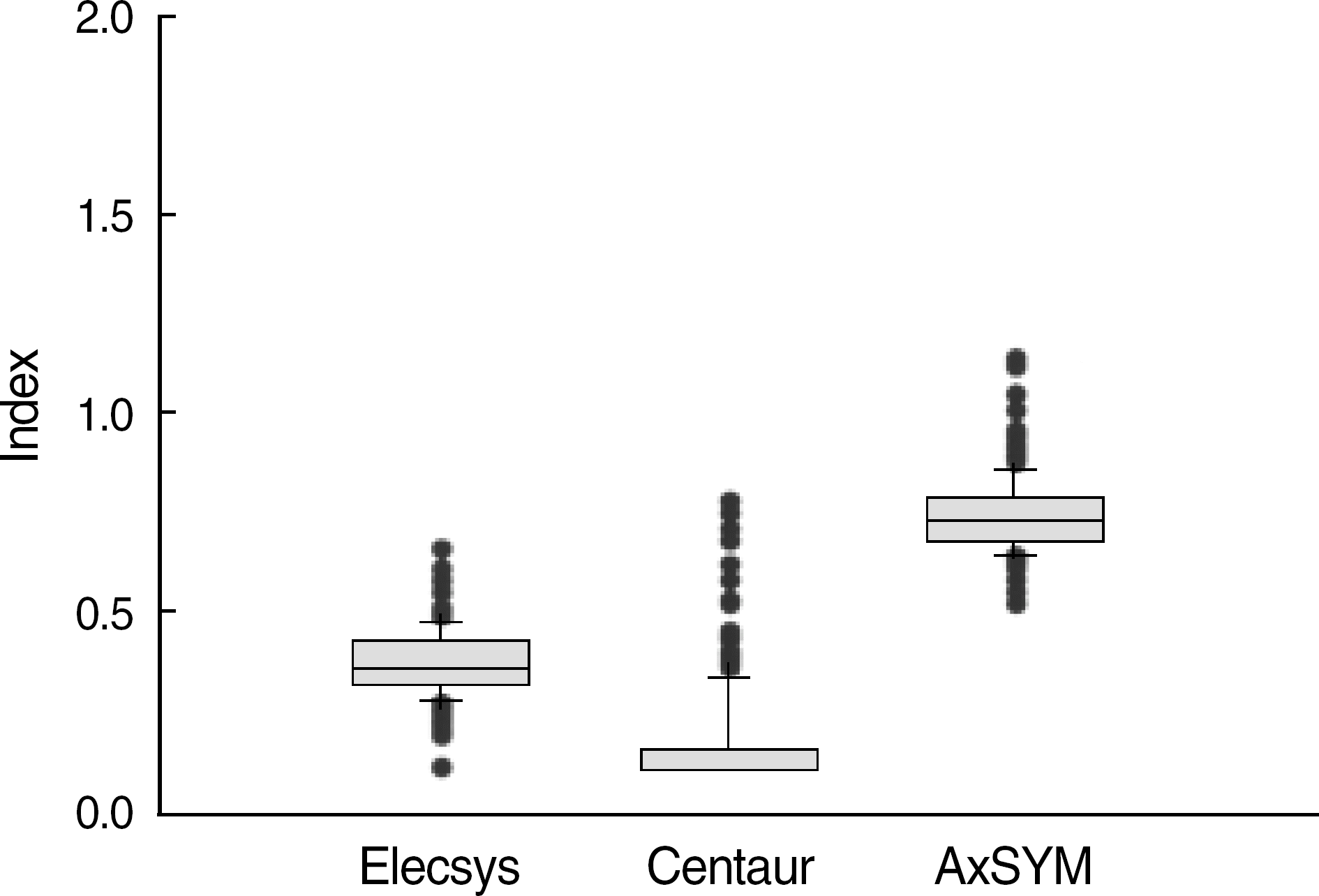
Fig. 2.
Distributions for positive HBsAg results (n=249). For the calculation of Spearman correlation coefficients, 196 samples with the index value of more than 1,000 with Centaur were excluded. (A) Elecsy vs AxSYM (R=0.44), (B) AxSYM vs Centaur (R=0.76), (C) Elecys vs Centaur (R=0.71).
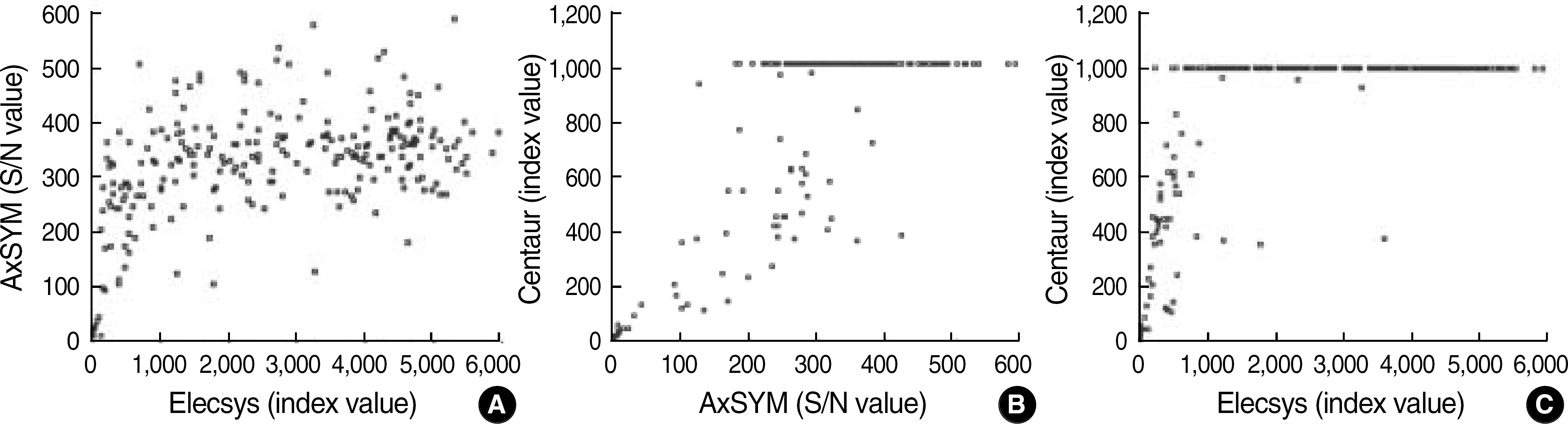
Table 1.
Characteristics of 3 kinds of automated HBsAg screening assays
| Test | Manufacturer | Test principle | Sample volume (L) |
Interpretation (signal/cutoff) |
|
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Elecsys HBsAg assay | Roche | ECLIA | 50 | ≥1 | <1 |
| ADVIA Centaur HBsAg assay | Bayer | CLIA | 100 | ≥1 | <1 |
| AxSYM HBsAg assay | Abbott | MEIA | 190 | ≥1 or S/N≥2* | <1 or S/N<2* |
Table 2.
Concordance among Elecsys, AxSYM, and Centaur HBsAg assays
| Sample group | No. of cases (%) | Percentage of concordance |
|---|---|---|
| Overall | 425 (100.0) | 100% |
| Negative | 176 (41.4) | 100% |
| Positive | 249 (58.6) | 100% |
Table 3.
Characteristics of 6 patients with low positive results below 10 (index value) in any one of the 3 assay systems
| Case No. | Diagnosis | HBV history | Elecsys (Index) | Centaur (Index) | AxSYM (S/N) | HBsAg Confirm* | HBeAg | Anti-HBc | Anti-HBs | bDNA (U/mL)† |
|---|---|---|---|---|---|---|---|---|---|---|
| 323 | Uterine myoma | − | 3.4 | 2.8 | 2.4 | + | − | + | − | <357 |
| 382 | Chronic hepatitis | +‡ | 7.4 | 4.2 | 3.7 | + | − | + | − | <357 |
| 434 | Abortion, unknown cause | − | 3.6 | 5.9 | 6.0 | + | − | − | − | 800 |
| 331 | Alcoholic liver cirrhosis | − | 10.9 | 12.4 | 7.0 | + | − | + | − | 366 |
| 349 | Spinal stenosis | − | 106.5 | 34.2 | 9.2 | + | − | + | − | 879 |
| 350 | Chronic tonsilitis | − | 11.0 | 46.9 | 7.1 | + | − | + | − | 106,549 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download