Abstract
Background
Recurrent spontaneous abortion (RSA) is defined as the occurrence of three or more consecutive spontaneous abortion before 20 gestational weeks. But, 40–50% of RSA still remain “unexplained”. Cytokines seem to play a critical role in the pathogenesis of unexplained RSA, and Th1 cytokines have been shown to exert deleterious effects on pregnancy. NK cytotoxicity has been reported to be predictive of subsequent abortion in women who had unexplained recurrent abortions. The aim of this study was to investigate immunophenotypic characteristics of peripheral blood mononuclear cells and evaluate Th1 cytokine (TNF-α) production in women with RSA.
Methods
The study group comprised 93 women with RSA, and the control group consisted of 40 healthy pregnant women. The population of CD56/CD16 cells was observed by using a two-color scattergram in FACScan (Becton Dickinson, San Jose CA, USA). Concentration of TNF-α was measured by an enzyme-linked immunoabsorbant assay (ELISA) using commercial kits (NEOGEN corporation, Lexington KY, USA).
Results
The percentage of CD56+/CD16-cells were significantly higher (P<0.05) in the patients with RSA (13.40±7.95%) than in the pregnant control group (9.12 ± 3.93%). We observed a significantly higher level of TNF-α (medians: 85.59 ± 8.29 pg/mL versus 44.80 ± 9.78 pg/mL; P<0.05) in RSA women compared to controls.
References
1. Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000; 14:839–54.

2. Druckmann R, Druckmann MA. Progesterone and the immunology of pregnancy. J Steroid Biochem Mol Biol. 2005; 97:389–96.

3. Laird SM, Tuckerman EM, Cork BA, Linjawi S, Blakemore AI, Li TC. A review of immune cells and molecules in women with recurrent miscarriage. Hum Reprod Update. 2003; 9:163–74.
4. Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunol Today. 1997; 18:478–82.
5. Choudhury SR, Knapp LA. Human reproductive failure I: immunological factors. Hum Reprod Update. 2001; 7:113–34.

6. Hill JA, Polgar K, Anderson DJ. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortions. J Am Med Assoc. 1995; 273:1933–6.
7. King A, Hiby SE, Verma S, Burrows T, Gardner L, Loke YW. Uterine NK cells and trophoblast HLA class I molecules. Am J Reprod Immunol. 1997; 37:459–62.

8. Aoki K, Kajiura S, Matsumoto Y, Ogasawara M, Okada S, Yagami Y, et al. Preconceptional natural-killer-cell activity as a predictor of miscarriage. Lancet. 1995; 345:1340–2.

9. Coulam CB, Goodman C, Roussev RG, Thomason EJ, Beaman KD. Systemic CD56+ cells can predict pregnancy outcome. Am J Reprod Immunol. 1995; 33:40–6.

10. Yamada H, Kato EH, Kobashi G, Ebina Y, Shimada S, Morikawa M, et al. High NK cell activity in early pregnancy correlates with subsequent abortion with normal chromosomes in women with recurrent abortion. Am J Reprod Immunol. 2001; 46:132–6.

11. Ruiz JE, Kwak JY, Baum L, Gilman-Sachs A, Beaman KD, Kim YB, et al. Intravenous immunoglobulin inhibits natural killer cell activity in vivo in women with recurrent spontaneous abortions. Am J Reprod Immunol. 1996; 35:370–5.
12. Ramhorst R, Agriello E, Zittermann S, Pando M, Larriba J, Irigoyen M, et al. Is the paternal mononuclear cells' immunization a successful treatment for recurrent spontaneous abortion? Am J Reprod Immunol. 2000; 44:129–35.

14. Moller MJ, Kammerer R, von Kleist S. A distinct distribution of natural killer cell subgroups in human tissues and blood. Int J Cancer. 1998; 78:533–8.
15. Ntrivalas EI, Kwak-Kim JY, Gilman-Sachs A, Chung-Bang H, Ng SC, Beaman KD, et al. Status of peripheral blood natural killer cells in women with recurrent spontaneous abortions and infertility of unknown aetiology. Hum Reprod. 2001; 16:855–61.

16. Day CP, Grove J, Daly AK, Stewart MW, Avery PJ, Walker M. Tumour necrosis factor-alpha gene promoter polymorphism and decreased insulin resistance. Diabetologia. 1998; 41:430–4.

17. King A, Wellings V, Gardner L, Loke YW. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol. 1989; 24:195–205.

18. Lachapelle MH, Miron P, Hemmings R, Roy DC. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol. 1996; 156:4027–34.
19. Vassiliadou N, Searle RF, Bulmer JN. Elevated expression of activation molecules by decidual lymphocytes in women suffering spontaneous early pregnancy loss. Hum Reprod. 1999; 14:1194–200.

20. Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship; is successful pregnancy a TH2 phenomenon? Immunol Today. 1993; 14:353–6.

21. Ferry BL, Sargent IL, Starkey PM, Redman CW. Cytotoxic activity against trophoblast and choriocarcinoma cells of large granular lymphocytes from human early pregnancy decidua. Cell Immunol. 1991; 132:140–9.

22. Loke YW, King A. Immunology of human placental implantation: clinical implications of our current understanding. Mol Med Today. 1997; 3:153–9.
23. Hunt JS, Robertson SA. Uterine macrophages and environmental programming for pregnancy success. J Reprod Immunol. 1996; 32:1–25.

24. Carp HJ, Toder V, Mashiach S, Nebel L, Serr DM. Recurrent miscarriage: a review of current concepts, immune mechanisms, and results of treatment. Obstet Gynecol Surv. 1990; 45:657–69.
25. Gilstrap LC 3rd, Hauth JC, Bell RE, Ackerman NB Jr, Yoder BA, Delemos R. Survival and short-term morbidity of the premature neonate. Obstet Gynecol. 1985; 63:37–41.
26. Arslan E, Colakoglu M, Celik C, Gezginc K, Acar A, Capar M, et al. Serum TNF-α, IL-6, Lupus anticoagulant and anticardiolipin antibody in women with and without a past history of recurrent miscarriage. Arch Gynecol Obstet. 2004; 270:227–9.
27. Lockwood CJ, Romero R, Feinberg RF, Clyne LP, Coster B, Hobbins JC. The prevalence and biologic significance of lupus anticoagulant and anticardiolipin antibodies in a general obstetric polulation. Am J Obstet Gynecol. 1989; 161:369–73.
Fig. 1.
Two color flow cytometric analysis of peripheral mononuclear cells from recurrent spontaneous abortion and control individuals. The X-axis represents the CD56-PE and the y-axis the CD16-FITC. The percentage in the upper right represents the CD56+/CD16+ mononuclear cells, and the lower right quadrant represents the percentage of CD56+/CD16-mononuclear cells. Abbreviation: RSA, recurrent spontaneous abortion.
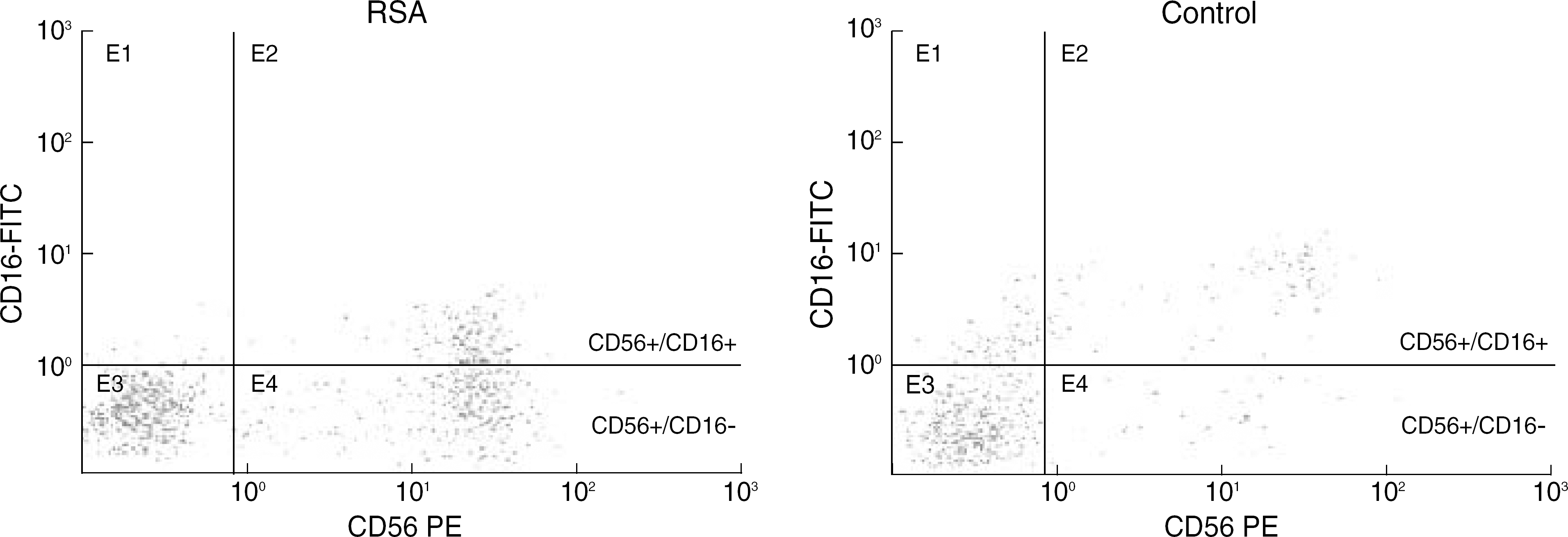
Fig. 2.
Distribution of TNF-alpha concentration in controls individuals and patients with recurrent spontaneous abortion. Median is indicated on the graph with a line.
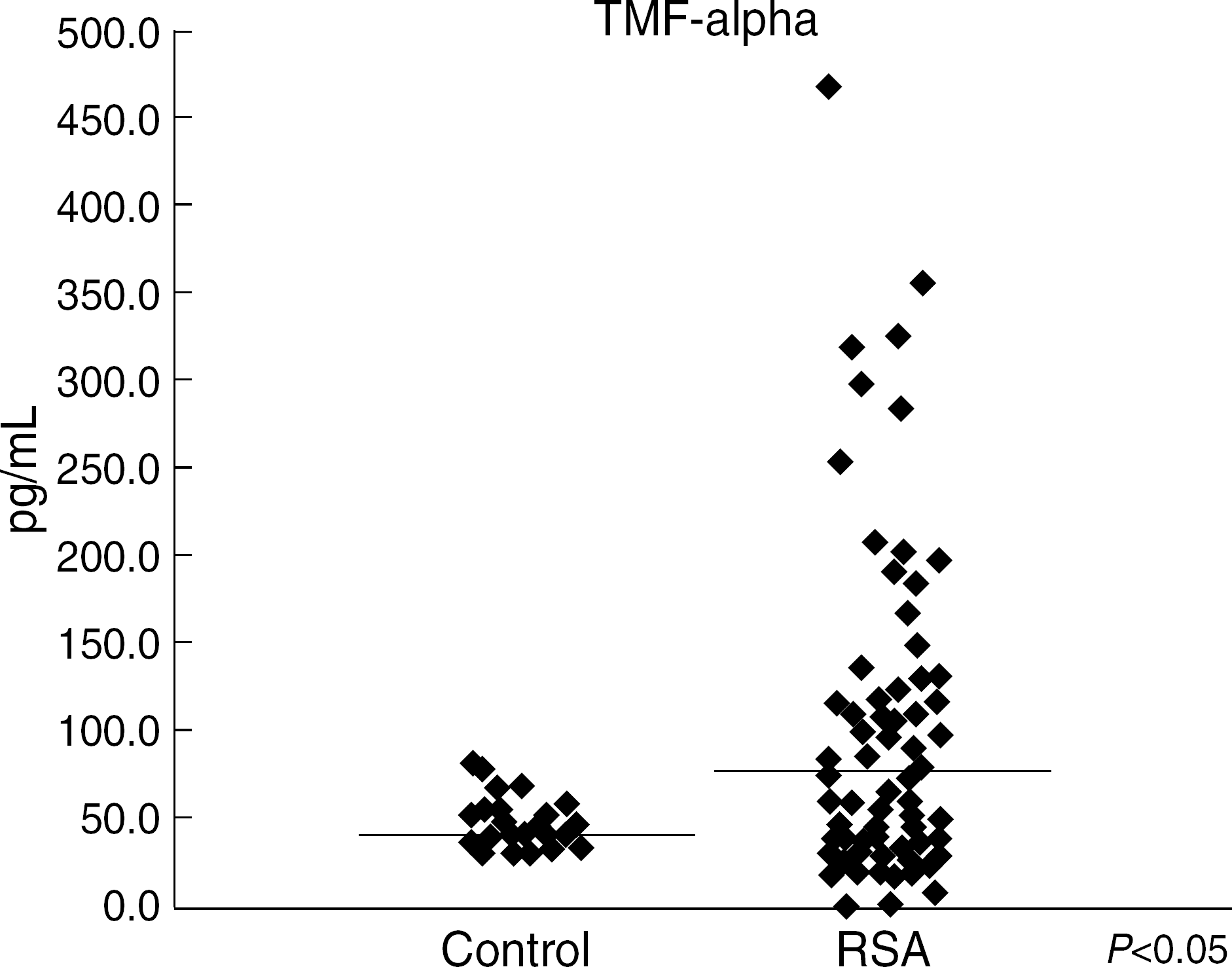
Table 1.
Mononuclear cells in patients with a history of recurrent abortions and in control individuals
| Mononuclear cells | RSA patients (n=93) % positive MN cells | Controls (n=40) % positive MN cells | Significance (P)† |
|---|---|---|---|
| CD56+ | 23.78±8.59* | 23.60±6.33 | NS |
| CD16+ | 12.38±6.73 | 19.39±8.30 | NS |
| CD56+/CD16+ | 10.40±6.61 | 14.35±5.21 | P<0.05 |
| CD56+/CD16- | 13.40±7.95 | 9.12±3.93 | P<0.05 |
| CD56-/CD16+ | 1.99±1.35 | 5.06±4.27 | NS |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download