Abstract
Background
Bone markers can provide a prognostic information about the risk of osteoporotic fracture and are useful tools for monitoring the efficacy of antiresorptive therapy. We evaluated the analytical performance of the bone markers of Elecsys 2010 (Roche Diagnostics Corp., Indianapolis, USA).
Methods
We evaluated the analytical performance of the Elecsys 2010 for serum parathyroid hormone (PTH), osteocalcin, and serum bone-derived degradation products of type I collagen C-telopeptide (S-CTX) using control material and patients' specimens. For the comparison studies, an immuno-radiometric assay was used for PTH and an ELISA for serum osteocalcin and serum bone-derived degradation products of type I collagen N-telopeptide (S-NTX). We established the reference intervals of S-CTX and serum osteocalcin by analyzing 4569 Korean healthy subjects according to sex and age.
Results
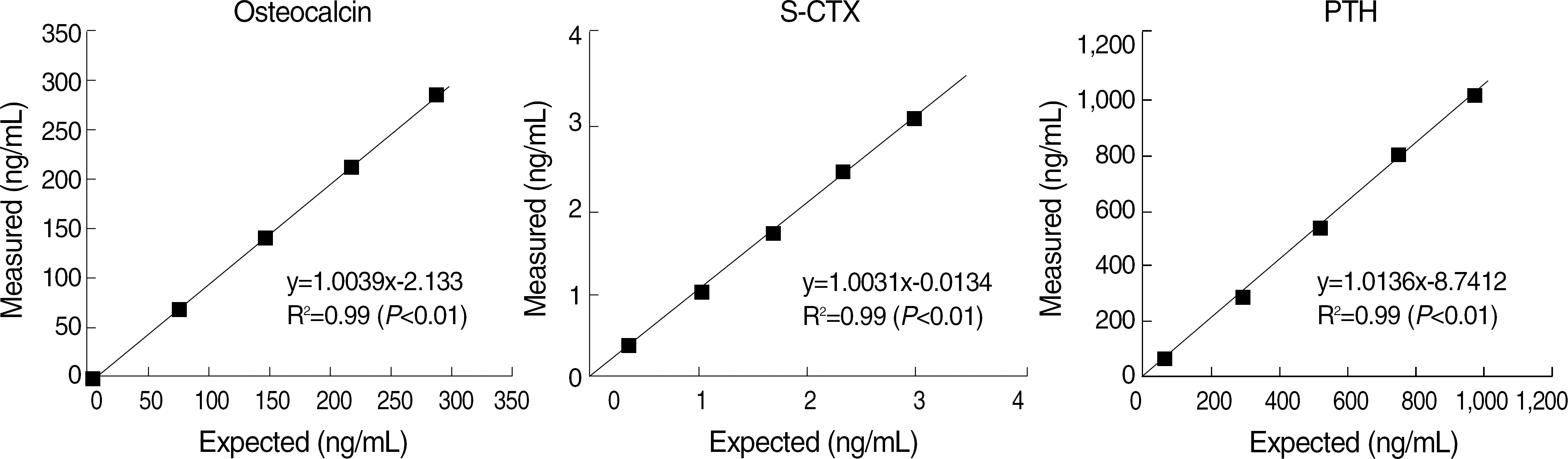
Within-run and total CV of most items were below 5% except S-CTX low level (5.42%). Elecsys 2010 showed a good linearity (r ≥0.99, P<0.01). Good correlations with other methods were found in osteolcalcin (r=0.95, P<0.01) and PTH (r=0.96, P<0.01). S-CTX showed a good correlation with S-NTX (r=0.76, P<0.01). Reference intervals of serum osteocalcin (ng/mL) and S-CTX (ng/mL) were 9.58–33.62 and 0.18–0.89, respectively, in adult male, 8.00–31.46 and 0.11–0.81 in 31–50 years old female, and 8.30–43.50 and 0.11–1.00 in 51–80 years old female.
Go to : 
References
1. Robert M, David F, Jennifer K, et al. Osteoporosis. 2nd ed.San Diego: Academic press;2001. p. 433–57.
2. Ebeling PR, Akesson K. Role of biochemical markers in the management of osteoporosis. Best Pract Res Clin Rheumatol. 2001; 15:385–400.

3. Miller PD, Baran DT, Bilezikian JP, Greenspan SL, Lindsay R, Riggs BL, et al. Practical clinical application of biochemical markers of bone turnover: Consensus of an expert panel. J Clin Densitom. 1999; 2:323–42.
4. World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis, Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994; 843:1–129.
5. Ray NF, Chan JK, Thamer M, Melton LJ III. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997; 12:24–35.

6. Gabriel SE, Tosteson AN, Leibson CL, Crowson CS, Pond GR, Hammond CS, et al. Direct medical costs attributable to osteoporotic fractures. Osteoporos Int. 2002; 13:323–30.

7. Price PA. Vitamin K-dependent formation of bone Gla protein and its function. Vitam Horm. 1985; 42:65–108.
8. Delmas PD. Biochemical markers of bone turnover. Acta Orthopaedica Scandinavica. 1995; 266:176–82.

9. Chesnut CH, Bell NH, Clark GS, Drinkwater BL, English SC, Johnston CC Jr, et al. Hormone replacement therapy in postmenopausal women: urinary N-telopeptide of type I collagen monitors therapeutic effect and predicts response of bone mineral density. Am J Med. 1997; 102:29–37.

10. Garnero P, Shi WJ, Gineyts E, Karpf DB, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994; 79:1693–700.

11. Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995; 333:1437–43.
12. Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997; 337:1641–7.

13. Braga de Castro Machado A, Hannon R, Eastell R. Monitoring alendronate therapy for osteoporosis. J Bone Miner Res. 1999; 14:602–8.

14. Eastell R, Mallinak N, Weiss S, Ettinger M, Pettinger M, Cain D, et al. Biological variability of serum and urinary N-telopeptides of type I collagen in postmenopausal women. J Bone Miner Res. 2000; 15:594–8.

15. Guillemant JA, Accarie CM, de la Gueronniere V, Guillemant SE. Different acute responses of serum type I collagen telopeptides, CTX, NTX and ICTP, after repeated ingestion of calcium. Clin Chim Acta. 2003; 337:35–41.

16. Garnero P, Borel O, Delmas PD. Evaluation of a fully automated serum assay for C-terminal cross-linking telopeptide of type I collagen in osteoporosis. Clin Chem. 2001; 47:694–702.

17. Hermse D, Franzson L, Hoffmann JP, Isaksson A, Kaufman JM, Leary E, et al. Multicenter evaluation of a new immunoassay for intact PTH measurement on the Elecsys System 2010 and 1010. Clin Lab. 2002; 48:131–41.
18. Jung TK, Kim HG, Choi HS, Lee NY, Park SG, Song KE. Evaluation of serum CTX and osteocalcin using Elecsys 2010. Korean J Clin Pathol. 2001; 21:459–64.
19. Iki M, Akiba T, Matsumoto T, Nishino H, Kagamimori S, Kagawa Y, et al. Reference database of biochemical markers of bone turnover for the Japanese female population. Japanese Population-based Osteoporosis (JPOS) Study. Osteoporos Int. 2004; 15:981–91.

20. Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996; 11:337–49.

21. Filip RS, Zagorski J. Age- and BMD-related differences in biochemical markers of bone metabolism in rural and urban women from Lublin Region, Poland. Ann Agric Environ Med. 2004; 11:255–9.
22. Chun SI, Ki CS, Kim SJ, Ahn JM, Kim DW, Kim JW. Serum osteocalcin and urine deoxypyridinoline levels in middle aged healthy Koreans; age and sex related variations. Korean J Clin Pathol. 1997; 17:244–51.
23. Chapurlat RD, Garnero P, Breart G, Meunier PJ, Delmas PD. Serum type I collagen breakdown product (serum CTX) predicts hip fracture risk in elderly women: the EPIDOS study. Bone. 2000; 27:283–6.
Go to : 
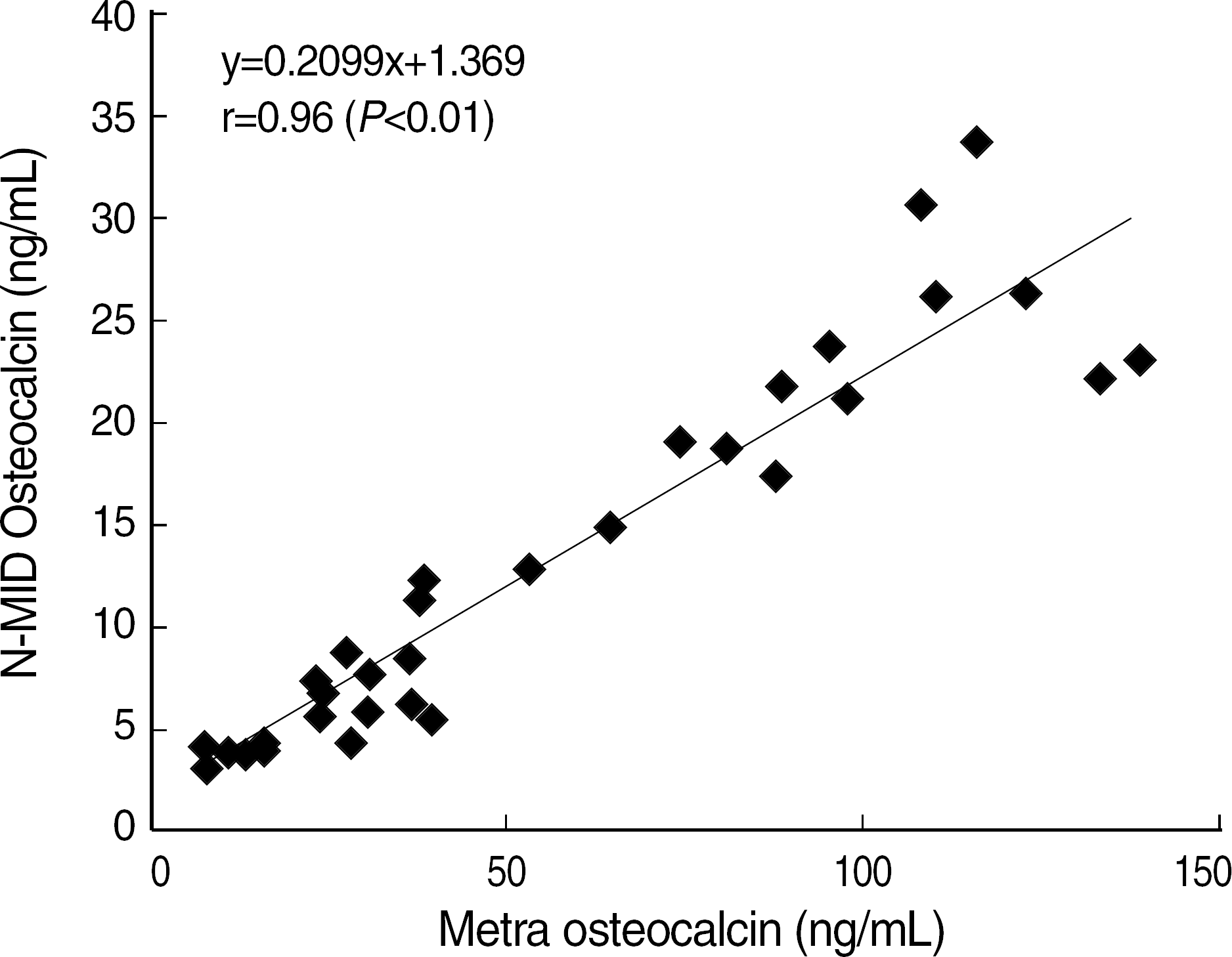 | Fig. 2.The correlation of N-MID osteocalcin by elecsys 2010 and metra osteocalcin by ELISA (n=40). |
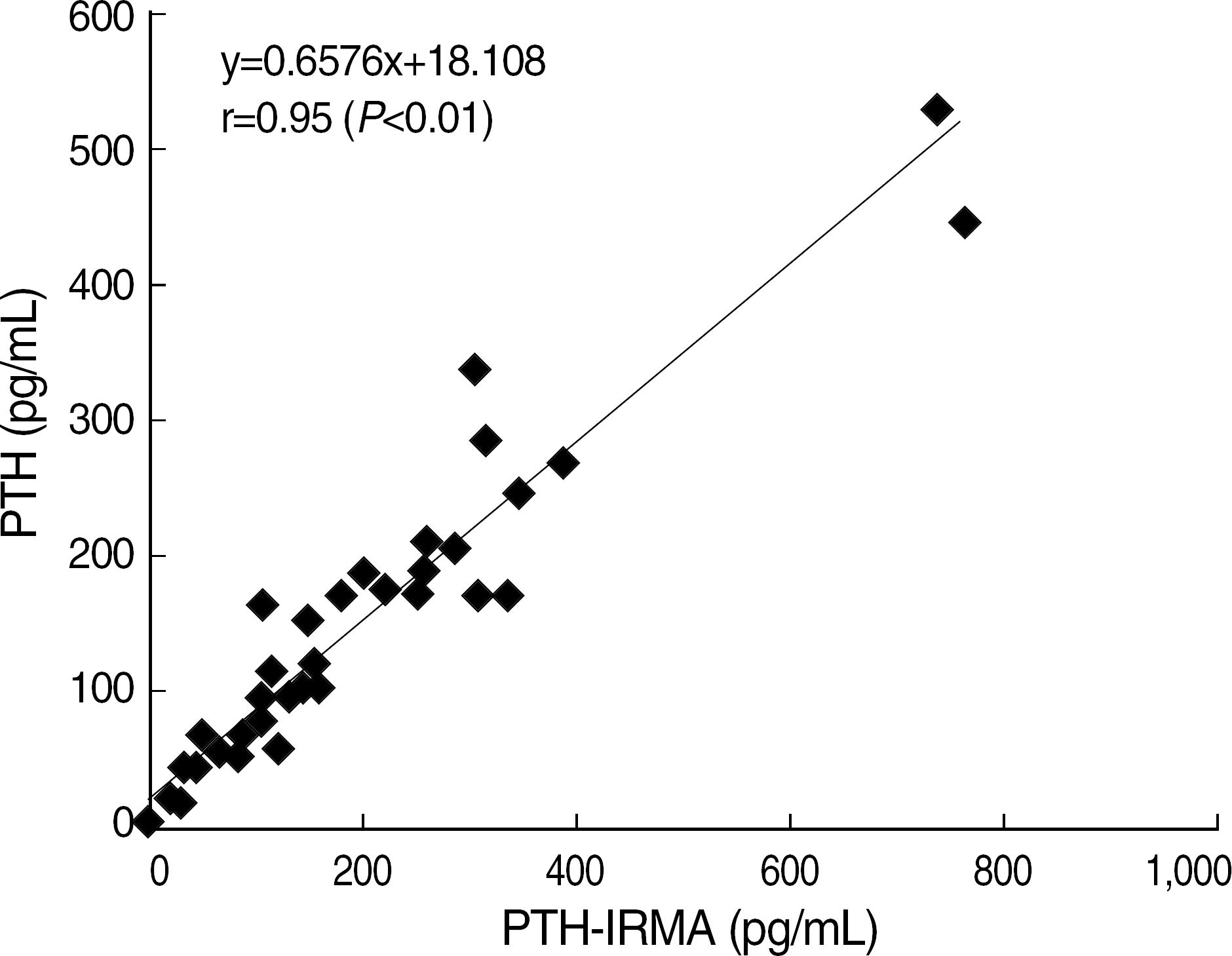 | Fig. 3.The correlation of PTH by elecsys 2010 and PTH-IRMA by immunoradiometric assay (n=40). |
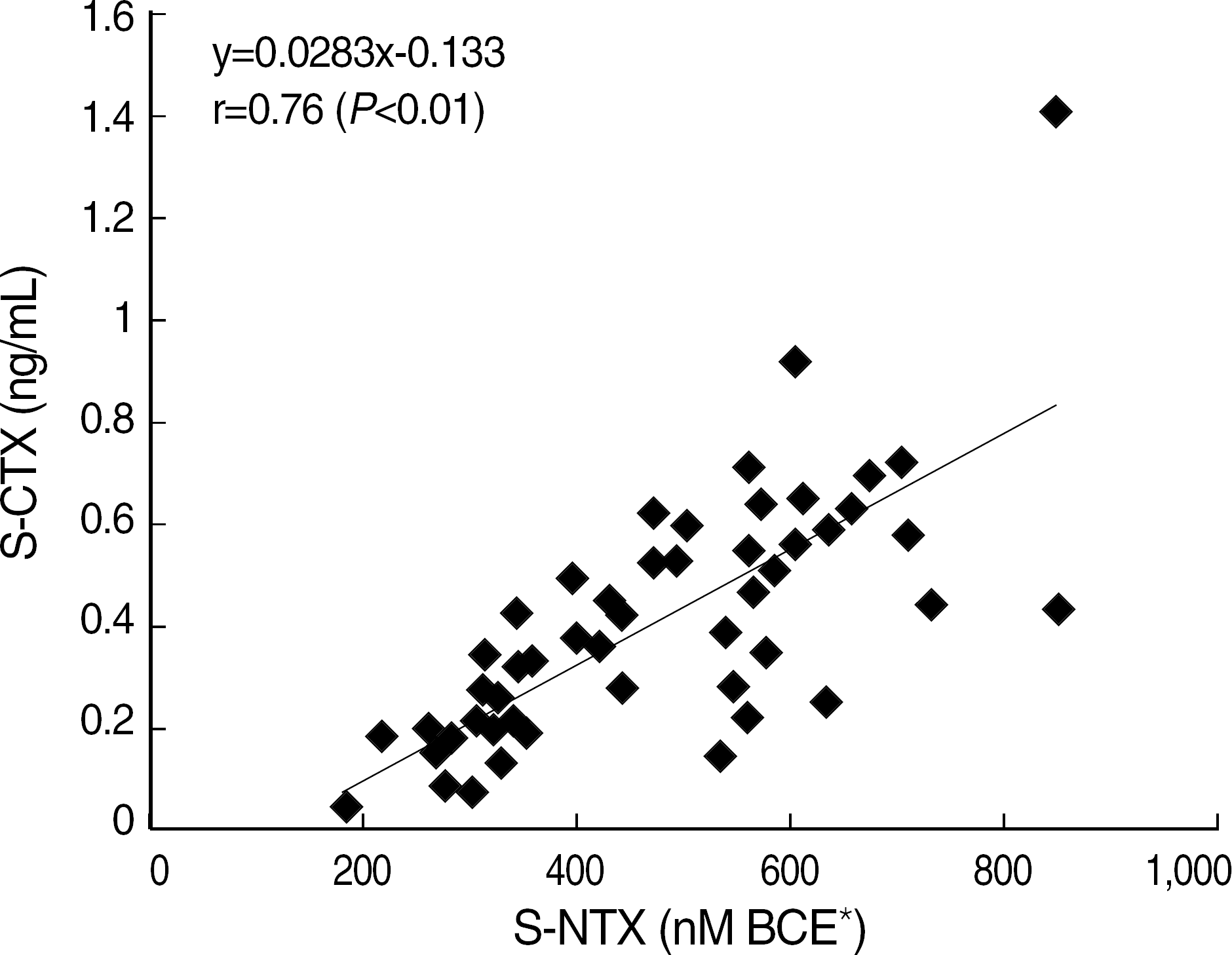 | Fig. 4.The correlation of S-NTX by ELISA and S-CTX by elecsys 2010 (n=58). *Nanomoles bone collagen equivalents. |
Table 1.
Within-run and total precision of elecsys 2010
Table 2.
Serum levels of osteocalcin (N-MID® Osteocalcin) and S-CTX (β-CrossLaps/serum) according to age and sex




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download