Abstract
Background
Glucometer is a most widely-used point-of-care testing (POCT) analyzer and plays an important role in diabetes management. We evaluated the performance of the recently developed glucometer, COSMOsensor (Cosmogenome Inc., Seoul, Korea), comparing it with three foreign-made glucometers.
Methods
COSMOsensor was evaluated for linearity, precision, comparison of method and analysing time as well as the effect of operator. Other glucometers, Accu-Chek inform (Roche Diagnostics LTD., Mannheim, Germany), Precision™PCx (Abbott Laboratories, Bedford, MA, USA), and Sure-Step®Flexx (LifeScan Inc., Milpitas, CA, USA) were evaluated for the same categories according to NCCLS guidelines.
Results
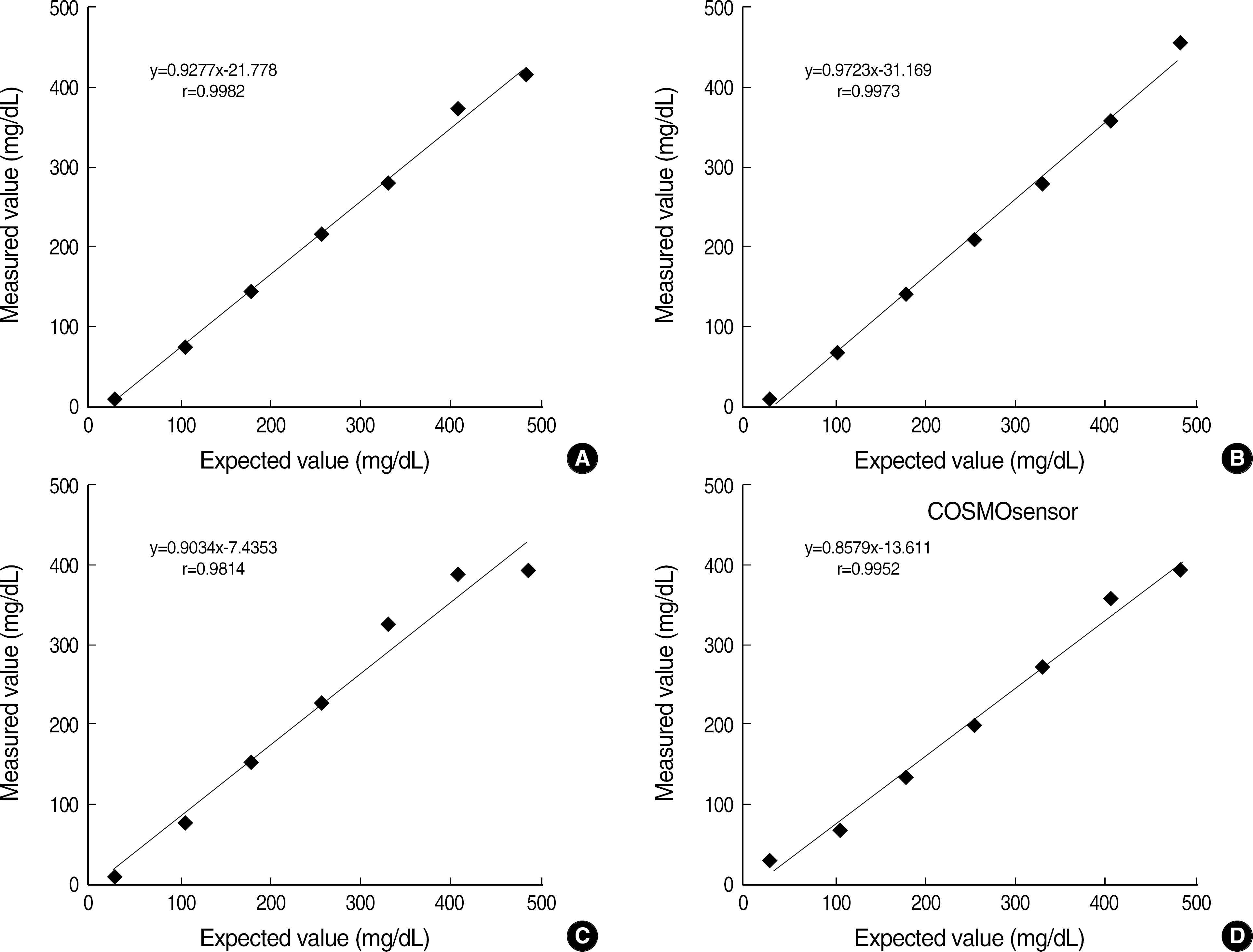
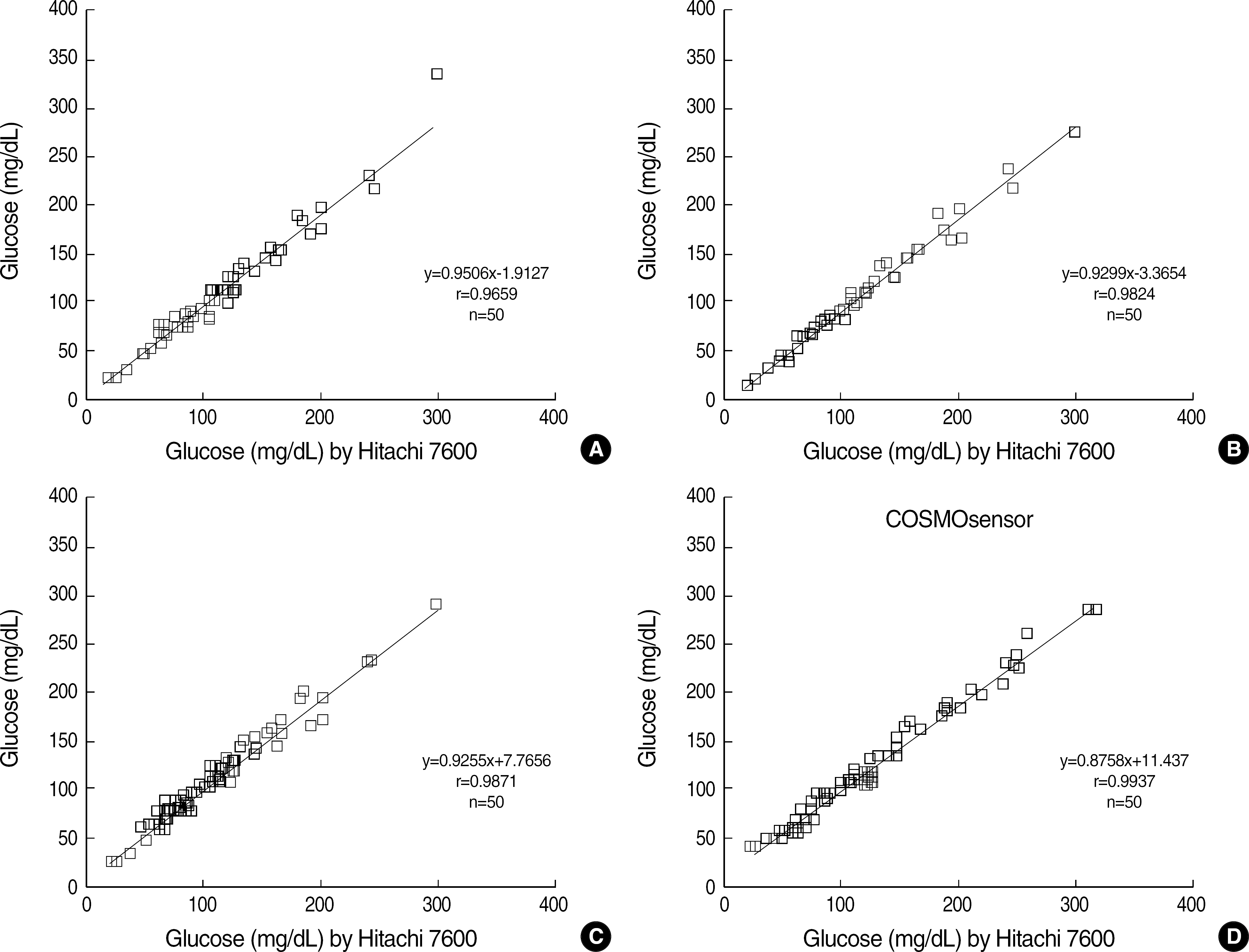
All four glucometers showed a good linearity (r≥0.9814) and the within-run and total-run coefficients of variation (CVs) were within 3.5%. A high correlation (r≥0.9659) was also found between the glucometers and Hitachi 7600 (Hitachi Co., Tokyo, Japan) in the central laboratory. Although differences with the reference method were within an allowable range, all glucometers showed variable bias compared with the reference method.
Conclusions
The COSMOsensor showed a good analytical performance in linearity, precision, and correlation with the reference method, when compared with other foreign-made glucometers. Its rapid turnaround time and easy operation are appropriate for diabetes management and a rapid POCT analyzer. All glucometers showed variable biases, which might be due to different calibration status.
References
1. Lee SY, Lee NY, Kim JW. Evaluation of 6 glucose testing system. Korean J Lab Med. 2003; 23:170–9.
2. Lee SY, Lee SG, Kim JW, Min WK, Park HS. Evaluation of Precision Q.I.DR glucose testing system. Korean J Clin Pathol. 1999; 19:425–32.
3. Woo JE, Lee DH, Hwang YS. Evaluation of Companion™ 2, home monitor of blood glucose using electrochemical electrode method. Korean J Clin Pathol. 1994; 14:309–16.
4. Min DW, Min WK, Hong KS. Evaluation of 3 monitors of blood glucose: Glucometer II, Reflolux II, Glucoscan-3000. J Lab Med Qual Assur. 1989; 11:245–52.
5. National Committee for Clinical Laboratory Standards. Evaluation of the linearity of quantitative analytical methods; Proposed guideline. Document EP6-P2.Vilanova, Pa: National Committee for Clinical Laboratory Standards;2001.
6. National Committee for Clinical Laboratory Standards. Evaluation of precision performance of clinical chemistry devices; Approved guideline. Document EP5-A.Vilanova, Pa: National Committee for Clinical Laboratory Standards;1999.
7. National Committee for Clinical Laboratory Standards. Method comparison and bias estimation using patient samples; Approved guideline. Document EP9-A.Vilanova, Pa: National Committee for Clinical Laboratory Standards;1995.
8. Kim CS, Jeong EK, Park J, Cho MH, Nam JS, Kim HJ, et al. Prevalence of diabetes mellitus (fasting plasma glucose by the ADA criteria and impaired fasting glucose according to anthropometric characteristics and dietary habits:1998 national health and nutrition survey. J Korean Diabetes Assoc. 2005; 29:151–66.
9. American Diabetes Association. Self-monitoring of blood glucose. Diabetes Care. 1996; 19:S62–6.
10. Skeie S, Thue G, Nerhus K, Sandberg S. Instruments for self-monitoring of blood glucose: comparisons of testing quality achieved by patients and a technician. Clin Chem. 2002; 48:994–1003.

11. Blake DR, Narthan DM. Point-of-care testing for diabetes. Point of Care. 2002; 1:155–64.
12. Guerci B, Drouin P, Grange V, Bougneres P, Fontaine P, Kerlan V, et al. Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance Intervention Active (ASIA) study. Diabetes Metab. 2003; 29:587–94.

13. Park CY, Ryu MS, Woo JT, Kim SW, Kim JW, Kim YS, et al. Evaluation of GlucoDr™ blood glucose testing system. Clinical Diabetes. 2002; 3:152–63.
14. Seo YH, Lee WS, Choi TY, Ahn YH. Clinical and laboratory assessment of a new monitor of blood glucose (Gluco-X). J Lab Med Qual Assur. 1993; 15:85–90.
15. Price CP, Hick JM, editors. ed.Point-of-care testing. 1st ed.Washington: American Association for Clinical Chemistry, Inc.;1999.
16. Knudson PE, Weinstock RS, Henry JB. Carbohydrates. Henry JB, editor. Clinical diagnosis and management by laboratory methods. 20th ed.Philadelphia: WB Saunders;2001. p. 211–23.
17. National Committee for Clinical Laboratory Standards. Ancillary (bedside) blood glucose testing in acute and chronic care facilities; Approved guidelines. Document C30-A.Vilanova, Pa: National Committee for Clinical Laboratory Standards;1994.
Fig. 3.
Bias plots of the difference (mg/dL) and % difference against the means between glucometers (A, B, C, or COSMOsensor) and Hitachi 7600.
Table 1.
Precision of 4 glucometers at 2 glucose concentration by single operator
Table 2.
Precision of 4 glucometers at 2 glucose concentration by multiple operators




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download