Abstract
Purpose
Free-floating thrombus (FFT) of the aorta is very rare but has a high risk of distal embolization. While the necessity of treating such a condition is evident, the diagnostic and therapeutic modalities remain controversial. Thus, we reviewed seven cases of FFT of the aorta.
Methods
A retrospective study was performed usings even patients diagnosed with FFT of the aorta at the Catholic University of Korea between January 1999 and December 2008. We excluded those patients who had thrombi with concomitant atherosclerotic or aneurysmal aorta.
Results
The mean patient age was 59.6±13.6 years old. The male-to-female ratio was 3:4. Embolization to arteries of the extremities occurred in two patients and to visceral arteries in four patients. Of these seven patients, four were initially treated with anticoagulation, and two were initially treated with thrombectomy; one patient refused any kind of treatment. Of the four patients treated with anticoagulation, three experienced complete dissolution of the thrombi while anticoagulation proved ineffective in the remaining patient who subsequently underwent thrombectomy. In all of the three patients who had received thrombectomy, postoperative anticoagulation was employed. There was no recurrence of FFT of the aorta during the follow-up period.
FFT of the aorta is defined as a nonadherent part of the thrombus floating within anormal aortic lumen.(1) This is a very rare condition with approximately 100 such cases described in the literature,(2) and is a disease entity different from the thrombus of anatherosclerotic aorta.(3) In general, it is unusual to find an aortic thrombus in a nonaneurysmal and non-atherosclerotic aorta.(3) FFT of the aorta is usually associated with hypercoagulable disordersin addition to its association with malignancy, trauma, and instrumentation.(3-9) Transient hypercoagulable states and turbulent blood flow have been described as the pathologic mechanisms.(10) Clinical presentations range from asymptomatic disease to symptoms related to cerebral, peripheral, or visceral embolization.(11) But, embolization of nonfloating and floating thombus is reported in 12% and 75% of patients, respectively.(12) There has been a growing interest in the understanding of this potential source of embolization and the defining of proper diagnostic and therapeutic approaches. We evaluated patients with FFT of the aorta to define the clinical presentation, proper diagnosis, and therapy of FFT of the aorta as anunusual source of peripheral embolization. This research was approved by the Catholic Medical Center Central Institute Review Board (CMC Central IRB) of the School of Medicine of the Catholic University of Korea.
A retrospective review of seven consecutive patients who were diagnosed with FFT of the aorta at the Catholic University of Korea between January 1999 and December 2008 was performed. We included just seven patients diagnosed with floating thrombi in the nonatherosclerotic and nonaneurysmal aorta by computed tomography angiography (CTA) or operative findings. Our principle of management for FFT of the aorta was utilizing systemic anticoagulation with LMWH as first-line therapy, but if the thrombus persisted or recurrent embolism occurred while receiving anticoagulation therapy, surgery was undertaken. After managements for the FFT of the aorta, the follow-up was done with using the CTA at 1, 3, 6 months, and annually thereafter. Patients were identified with vascular registries and medical records. The following clinicopathological data were retrieved: age, sex, hypercoagulability (protein C, protein S, antithrombin III, factor Va Leiden mutation, anticardiolipin antibody, and homocysteine), clinical presentation, diagnostic modality, treatment, and prognosis. Data were presented as mean±standard deviation.
Seven patients were diagnosed with FFT of the aorta. Of those, three were male and four were female, the mean age was 59.6±13.6 years (Table 1). Six patients were symptomatic, and one patient was asymptomatic. Among the six patients who were symptomatic, one patient had pallor and coldness in the left upper extremity, another patient had left calf pain, and the other four patients had sudden abdominal pain. The FFT of the aorta was located on the aortic arch and abdominal aorta in one patient, descending thoracic aorta in two patients, and abdominal aorta in four patients (Table 1). Among the seven patients, three patients had hypercoagulable disorders. One patient had decreased activity of Protein S, another patient had decreased activity of Protein C and S, and the last patient had antithrombin-III (AT-III) deficiency. The remaining four patients did not have hypercoagulable disorders (Table 1). Complications of the FFT of the aorta consisted of upper limb ischemia in one patient, lower limb ischemia in one patient, renal infarction in one patient, superior mesenteric artery occlusion in one patient, and splenic infarction in two patients (Table 1). In all patients, electrocardiograms (ECGs) showed regular sinus rhythms, and echocardiographies showed no regional wall motion abnormalities and no cardiac thrombus. These enabled us to exclude the possibility of cardiogenic embolization. In five patients who had FFTs in the abdominal aorta, three patients had it in the suprarenal abdominal aorta, one patient had it in the juxtarenal abdominal aorta, and the last patient had it in the infrarenal abdominal aorta. In three patients who had the FFTs in the suprarenal abdominal aorta, one patient had simultaneous FFTs in the aortic arch and suprarenal abdominal aorta, and had the embolization in left upper extremity. The second patient had the embolization in the spleen, and the last patient had the embolization in the superior mesenteric artery (SMA). In one patient who had the FFT in the juxtarenal abdominal aorta, there was no distal embolization. In one patient with the FFT in the infrarenal abdominal aorta, the embolization was in left lower extremity. Initial treatment consisted of anticoagulation with low molecular weight heparin (LMWH) 1 mg/kg subcutaneously twice a day in four patients, and thrombectomy with postoperative anticoagulation in two patients (Table 1). Initially, anticoagulation was via administration of LMWH for two weeks, followed by wafarin with accommodating International Normalized Ratio (INR) from two to three weeks. One patient refusedany kind of treatment. Among the four patients treated initially with anticoagulation for two weeks, there was complete dissociation of the thrombus in three patients (Fig. 1), and no change of the thrombus in one patient. We conducted thrombectomy and postoperative anticoagulation for this patient who showed no change in FFT after initial anticoagulation (Fig. 2). The operative findings of all of three patients showed that there was just the FFT arising from the lumbar artery, and no atherosclerotic or aneurysmal change in the aorta (Fig. 2). Morphologically, all of the FFTs removed from three patients had irregular margins and were whitish thrombi. The mean dimension of the removed FFTs was 3.3×1.7×1.2 centimeters (cm). Surgical treatments for FFT of the aorta of three patients were thrombectomies (Fig. 2). One patient had no complication of the FFT but had a thrombectomydone; the Whipple's operation was done on him four weeks prior, and the computed tomography (CT) was checked due to abdominal distension and pain on the twenty-eighth postoperative day. The CT showed a huge cyst in the peripancreatic area and an incidental FFT of the abdominal aorta. We performed open drainage of the cyst and simultaneousthrombectomy for the FFT of the abdominal aorta to prevent distal embolization. The patient who had refused treatment expired suddenly on the fifth hospital day. Follow-up periods for the other six patients ranged from 1 month to 96 months (30.0±34.0) (Table 1). We maintained anticoagulation therapy during the follow-up period, and there was no reccurrence of the FFT of the aorta. Among the six patients who had distal embolization, one patient refused treatment and expired on the fifth hospital day, and distal embolizations of the other five patients were completely cured and did not recur during the follow-up period. On Table 1, "prognosis" reflects whether the FFT and the distal embolizationrecurs or not. Thus, good prognosis means that there is no recurrence of the FFT and distal embolization. During follow-up, two patients expired. One patient expired suddenly on the fifth hospital day, and the other patient expired due to postoperative pneumonia and sepsis on the twenty-sixth postoperativeday.
Of the four patients treated initially with anticoagulation, we witnessed complete dissociation of the thrombus in three patients, while there was no change of thrombus in one patient for whom thrombectomy and postoperative anticoagulation were performed. All of the four patients had no recurrence of FFT and distal embolization during the follow-up period. Of the two patients treated initially with thrombectomy, one patient had no recurrence of FFT and distal embolization, but the other patient expired during the follow-up period.
FFT in the aorta is an uncommon and dangerous condition.(13) The thrombus can disintegrate and shed arterial emboli which may manifest clinically as visceral and peripheral events.(13) Because of the potential risk of distal embolization, treatment is mandatory.(13) The natural history of such FFT is not well known.(1) In the literature,(14) many cases of FFT of the aorta are those thrombi that originated from atheromatous plaques. So, we have searched for cases of FFT of the nonaneurysmal and nonatheroscleroticaorta on the web. Table 2 shows reports describing FFT of the nonaneurysmal and nonatheroscleroticaorta since 2008. In the reports, except our cases in Table 2, the location of thrombi was the ascending thoracic aorta in three cases (50.0%), CTA was the preferred diagnostic modality in all three cases (50.0%), Surgical treatments were employed in all cases (100%), Embolization occurred in five cases (83.3%), and the locations of embolization were the arteries of the upper extremities in three cases (60.0%). However, in our cases, the locations of the FFTs in five cases (71.4%) were the abdominal aorta, the diagnostic modality of all cases was CTA, anticoagulation was preferred as the first choice of treatment, embolization occurred in six cases (85.7%), and locations of embolizations were visceral arteries in four cases (66.7%).
In general, it is unusual to find aortic thrombus formation in a nonaneurysmal and nonatherosclerotic aorta.(3) In these cases, it is usually associated with hypercoagulable disorders, such as hyperhomocysteinemia, combined protein C and protein S deficiency, elevated levels of clotting factor VIII, antithrombin III deficiency, polycytemiavera, and systemic lupus erythematosus, in addition to its association with malignancy, trauma, and instrumentation.(3-9) Transient hypercoagulable states and turbulent blood flow have been described as mechanistic etiologies.(10) Among our seven patients, all of three patients who received surgical treatments had FFTs from just the lumbar artery and no the atherosclerotic or aneurysmal change in the aorta in operative findings, and they did not have any hypercoagulable disorders. Thus, we assumed turbulent blood flow as their etiology. Clinical presentation ranges from asymptomatic disease to symptoms related to cerebral, peripheral, or visceral embolization.(11) Many cases of aortic thrombi are diagnosed after an embolic event usually involving the extremities.(19) However, some cases are discovered coincidentally during routine examination of patients in whom migration has either not occurred or has involved an asymptomatic territory.(13)
As reported in the literature,(20) diagnosis of FFT is generally made using transesophageal echocardiography (TEE) but CTA can also be considered a valid diagnostic tool to evaluate not only the aorta but also the supra-aortic vessels and the pulmonary arteries. It is becoming more evident that TEE can be considered as an imaging procedure of choice for heart and thoracic aortic thrombosis.(21) The mid to distal aortic arch is sometimes poorly visualized on TEE, and may be better assessed using magnetic resonance angiography (MRA) or magnetic resonance imaging (MRI).(22) In other literature,(1) it was reported that CTA had replaced TEE as the diagnostic modality and planning tool of choice.
We believe that TEE is viable only for thrombus of heart and aortic arch in diagnosis of aortic thrombus, and is invasive and uncomfortable to patients. Also, we diagnosed all of the seven patients with FFT of the aorta by CTA. Thus, we recommend CTA as a valuable tool for diagnosis of floating thrombus of the aorta in tolerantpatients.
Without any doubt, the presence of thrombus implies the risk of embolization with potentially dire consequences.(13) Embolization ofnonfloating and floating thombus is reported in 12% and 75% of patients, respectively.(12) Thus these patients' therapy should focus on preventing the evolution of mobile lesions and provide protection against the embolic potential of these lesions.(13)
The treatment of FFT in the aorta remains highly controversial.(22) And, various treatment modalities have been described.(1) Medical treatments (anticoagulation and thrombolysis) may lead to complete dissolution of the thrombus in most patients,(1) and we have experienced complete dissociation of the thrombus in three patients. However, medical treatment has been reported to increase the risk of systemic embolization paradoxically by causing lysis of the pedicle more rapidly compared with the thrombus itself.(8) And, with regard to medical treatments, the optimal lapse of time for heparin therapy and the optimal dosage for wafarin anticoagulation therapy are still uncertain because no reports in the literature address these issues either for floating aortic thrombus or arterial thrombotic events in general.(23) Surgical treatments (Thrombectomy, thrombo-endarterectomy, and aortic replacement) require careful analysis of CTA as the diagnostic and planning tool,(1) and carry a high morbidity, mainly due to cerebral complication occurring in up to 29% of the patients.(24) Until now, there has been no obvious recommendation for postoperative anticoagulation of the floating thrombus of the aorta. But, we think that there was no recurrence of the thrombus in our patients owing to the postoperative anticoagulation. We suggest that postoperative anticoagulation is necessary for preventing the recurrence of the floating thrombus of the aorta, such as in our cases.
Exclusion by endovascular stent-graft has been recently suggested.(20) Similar to open surgery, stent-graft not only can exclude the thrombus but also treats the potential underlying cause of the thrombotic lesion by covering the atherosclerotic aortic wall.(20) Another benefit of this treatment is the possibility of combining the thoracic procedure with a peripheralembolectomy during the same surgical access.(20) Moreover, several reports have been published on percutaneousembolectomy performed with a "home-made" protection device to reduce the risk of distal embolization.(25-28) Criado et al.(20) reported the first use of an endograft to cover a recurrent floating thrombus located in the descending aorta. In their case, surgical thrombectomy had been performed one month earlier.(20) Since then, several authors have reported their experience with endograft implantation.(1)
The hybrid debranching and endovascular approach is a less invasive option compared toopen surgery.(1) It can also be applied to treat lesions of the thoracic and abdominal aorta simultaneously.(29) Although the risk of accidental embolization seems to be low, it likely cannot be eliminated completely, even with antegradeendograft implantation.(1) If embolization occurs, surgical embolectomy or thrombus aspiration can be performed with usual techniques.(1) Clear recommendations regarding anticoagulation regimens to be used before and after endograft exclusion of mobile thrombus of the thoracic aorta are lacking.(1)
In conclusion, because of the high risk of distal embolization from the floating thrombus of the aorta, treatment is mandatory. But, the treatment protocol remains highly controversial. Were commend systemic anticoagulation with LMWH as the first line of therapy for the floating thrombus of the aorta. However, if the thrombus persists or recurrent embolism occurs during anticoagulation therapy, surgery should be undertaken. Follow-up must include long-term anticoagulation therapy and routine surveillance using CTA since TEE is not only an invasive and uncomfortable modality to patients, but it also does not allow forevaluation of the entire aorta.
Figures and Tables
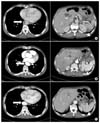 | Fig. 1CTA shows the complete dissociation of thrombus by anticoagulation therapy for the floating thrombus of the aorta.The A shows the CTA finding before anticoagulation therapy. The arrow indicates the floating thrombus of the descending thoracic aorta, and the arrow head indicates the splenic infarction. The B shows the CTA finding after anticoagulation for two weeks. The arrow indicates the complete dissociation of the floating thrombus of the descending thoracic aorta, and the arrow head indicates the healing of the splenic infarction. The C shows the CTA findings three months after initial anticoagulation was started. The arrow and the arrow head indicate that there is no reccurrence of the floating thrombus and the splenic infarction. |
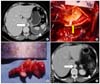 | Fig. 2CTA and operative findings show the case that thrombectomy and splenectomy have been done since anticoagulation was ineffective for the floating thrombus of the abdominal aorta and the splenic infarction. The A shows the CTA finding before treatment. The empty arrow indicates the floating thrombus of the abdominal aorta, and the arrow head indicates the splenic infarction. The B shows the intraoperative finding. The arrow indicates the floating thrombus arising from the lumbar artery. There is no atherosclerotic and aneurysmal change in the aorta. The C shows the removed thrombus. The length measures nearly 3.5 centimeter (cm). The D shows the CTA finding after thrombectomy and splenectomy. The empty arrow indicates that the floating thrombus of the abdominal aorta has been completely removed. The arrow head indicates that the splenectomy has been done. |
Table 2
Reports describing free floating thrombus (FFT) of the aorta since 2008

n = number; DTA = descending thoracic aorta; AsA = ascending thoracic aorta; AA = aortic arch; AbA = abdominal aorta; CTA = computed tomography angiography; MRA = magnetic resonance angiography; TEE = transesophageal echocardiography; EVT = endovascular treatment; Surg. = surgery; AC = anticoagulation; ext. = extremity.
References
1. Rancic Z, Pfammatter T, Lachat M, Frauenfelder T, Veith FJ, Mayer D. Floating aortic arch thrombus involving the supraaortic trunks: successful treatment with supra-aortic debranching and antegradeendograft implantation. J Vasc Surg. 2009. 50:1177–1180.
2. Choukroun EM, Labrousse LM, Madonna FP, Deville C. Mobile thrombus of the thoracic aorta: diagnosis and treatment in 9 cases. Ann Vasc Surg. 2002. 16:714–722.
3. Berneder S, Van Ingen G, Eigel P. Arch thrombus formation in an apparently normal aorta as a source for recurrent peripheral embolization. Thorac Cardiovasc Surg. 2006. 54:548–549.
4. Onwuanyi A, Sachdeva R, Hamirani K, Islam M, Parris R. Mutiple aortic thrombi associated with protein C and S deficiency. Mayo Clin Proc. 2001. 76:319–322.
5. Shapiro ME, Rodvien R, Bauer KA, Salzman EW. Acute aortic thrombosis in antithrombin III deficiency. JAMA. 1981. 245:1759–1761.
6. Josephson GD, Tiefenbrun J, Harvey J. Thrombosis of the descending thoracic aorta: a case report. Surgery. 1993. 114:598–600.
7. Hanson JA, Lloyd ME, Hughes GR. Aortic root thrombus causing stroke in a patient with systemic lupus erythematosus. Scand J Rheumatol. 1994. 23:156–158.
8. Geha AS, El-Zein C, Massad MG, Bagai J, Khoury F, Evans A, et al. Surgery for aortic arch thrombosis. Thorac Cardiovasc Surg. 2004. 52:187–190.
9. Agolini SF, Shah KT, Goodreau JJ, McLoughlin TM Jr, Sinclair MC. Splenic infarction caused by a large thoracic aortic thrombus. J Vasc Surg. 1997. 26:1069–1072.
10. Kalangos A, Baldovinos A, Vuille C, Montessuit M, Faidutti B. Floating thrombus in the ascending aorta: a rare cause of peripheral emboli. J Vasc Surg. 1997. 26:150–154.
11. Laperche T, Laurian C, Roudaut R, Steg PG. Mobile thromsoses of the aortic arch without aortic debris: a transesophageal echocardiographic finding associated with unexplained arterial embolism. Circulation. 1997. 96:288–294.
12. Karalis DG, Chandrasekaran K, Victor MF, Ross JJ Jr, Mintz GS. Recognition and embolic potential of intraaortic atherosclerotic debris. J Am Coll Cardiol. 1991. 17:73–78.
13. Piffaretti G, Tozzi M, Mariscalco G, Bacuzzi A, Lomazzi C, Rivolta N, et al. Mobile thrombus of the thoracic aorta: management and treatment review. Vasc Endovascular Surg. 2008. 42:405–411.
14. Choukroun EM, Labrousse LM, Madonna FP, Deville C. Mobile thrombus of the thoracic aorta: diagnosis and treatment in 9 cases. Ann Vasc Surg. 2002. 16:714–722.
15. Fanelli F, Gazzetti M, Boatta E, Ruggiero M, Lucatelli P, Speziale F. Acute left arm ischemia associated with floating thrombus in the proximal descending aorta: combined endovascular and surgical therapy. Cardiovasc Intervent Radiol. 2010. Epub ahead.
16. Pousios D, Velissaris T, Duggan S, Tsang G. Floating intraaortic thrombus presenting as distal arterial embolism. Interact Cardiovasc Thorac Surg. 2009. 9:532–534.
17. Madershahian N, Kuhn-Regnier F, Mime LB, Slottosch I, Langebartels G, Sindhu D, et al. A loose cannon: free-floating thrombus in ascending aorta. J Card Surg. 2009. 24:198–199.
18. Ryu YG, Chung CH, Choo SJ, Kim YS, Song JK. A case of antiphospholipid syndrome presenting with a floating thrombus in the ascending aorta. J Thorac Cardiovasc Surg. 2009. 137:500–502.
19. Sari I, Davutoglu V, Bayram N, Soydinc S. Fatal giant aortic thrombus presenting with pulmonary edema in a patient with chronic obstructive pulmonary disease. Clin Appl Thromb Hemost. 2008. 14:486–488.
20. Criado E, Wall P, Lucas P, Gasparis A, Proffit T, Ricotta J. Transesophageal echo-guided endovascular exclusion of thoracic aortic mobile thrombi. J Vasc Surg. 2004. 39:238–242.
21. Soleimani A, Marzban M, Sahebjam M, Shirani S, Sotoudeh-Anvari M, Abbasi A. Floating thrombus in the aortic arch as an origin of simultaneous peripheral emboli. J Card Surg. 2008. 23:762–764.
22. Sanon S, Phung MK, Lentz R, Buja LM, Tung PP, McPherson DD, et al. Floating, non-occlusive, mobile aortic thrombus and splenic infarction associated with protein C deficiency. J Am Soc Echocardiogr. 2009. 22:1419e1–e3.
23. Bowdish ME, Weaver FA, Liebman HA, Rowe VL, Hood DB. Anticoagulation is an effective treatment for aortic mural thrombi. J Vasc Surg. 2002. 36:713–719.
24. Gouëffic Y, Chaillou P, Pillet JC, Duveau D, Patra P. Surgical treatment of nonaneurysmal aortic arch lesions in patients with systemic embolization. J Vasc Surg. 2002. 36:1186–1193.
25. Vorwerk D, Günther RW, Schürmann K, Schmitz-Rode T, Biesterfeld S. Percutaneous balloon embolectomy with a self-expanding tulip sheath: in vivo experiments. Radiology. 1995. 197:153–156.
26. Vorwerk D, Günther RW, Schürmann K, Schmitz-Rode T. New device for wire and catheter capturing: in vivo experiments and first clinical experience. Cardiovasc Intervent Radiol. 1995. 18:312–314.
27. Vorwerk D, Schmitz-Rode T, Schürmann K, Tacke J, Guenther RW. Use of a temporary caval filter to assist percutaneous iliocaval thrombectomy: experimental results. J Vasc Interv Radiol. 1995. 6:737–740.
28. Vorwerk D, Guenther RW, Clerc C, Schmitz-Rode T, Imbert C. Percutaneousembolectomy: in vitro investigations of the self-expanding tulip sheath. Radiology. 1992. 182:415–418.
29. Zhang WW, Abou-Zamzam AM, Hashisho M, Killeen JD, Bianchi C, Teruya TH. Staged endovascular stent grafts for concurrent mobile/ulcerated thrombi of thoracic and abdominal aorta causing recurrent spontaneous distal embolization. J Vasc Surg. 2008. 47:193–196.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download