Abstract
Purpose
We investigated the correlation between expression of c-erbB-2 and p53 proteins and clinicopathologic features of gastric cancer to reveal prognostic factors.
Methods
125 patient records under going curative resection for gastric carcinoma at our institution from January 2000 to June 2003 were collected. Surgical specimens embedded in paraffin block were evaluated for p53 and c-erbB-2 protein as detected by immunohistochemistry.
Results
Samples from 30 cases (24.0%) of 125 patients with gastric adenocarcinomas demonstrated positive staining for c-erbB-2 and 72 patients (57.6%) showed positive nuclear staining for p53 protein. c-erbB-2 stained tumors were significantly associated with the depth of tumor invasion (P=0.022), lymph node metastasis (P=0.004) and lymphatic invasion (P=0.019). In a subgroup of patients with gastric carcinoma not exposed to serosa (n=91), expression of both c-erbB-2 and p53 significantly related with poor disease-free survival (OR=5.107) and survival (OR=4.449) in multivariate analysis.
Conclusion
When patients with gastric adenocarcinoma showed expression of c-erbB-2 with p53, they were associated with aggressive pathologic features. In the subgroup of patients with gastric adenocarcinoma that did not involve serosa, expression of c-erbB-2 combined with p53 could become a predictive factor for recurrence and survival in multivariate analysis.
Gastric cancer is one of the most common malignancies worldwide. It remains fatal in spite of early diagnosis and the development of chemotherapeutic agents to combat it.(1) The most important cause of death after curative treatment for locally advancced gastric cancer is recurrence of primary tumor. The recurrence may be ameliorated by appropriate adjuvant treatment using agents targeting specific molecules. The mechanism of new drug for target treatment would involve clarifying the mechanism of action of various genes associated with the carcinogenesis of gastric cancer. Before attempting to clarify the mechanism, confirming whether tumor expressions of specific oncogenes or tumor suppressor genes are associated with the clinicopathologic features of gastric cancer as well as the prognosis of patients, is important.
The c-erbB-2 gene is a proto-oncogene located on chromosome 17. It expresses HER2/neu protein, one of the epithelial growth factor receptor (EGFR) families, and has tyrosine kinase (TK) activity, which mediates cancer proliferation.(2) The p53 gene is also located on chromosome 17. It is a representative tumor suppressor gene, and mutations of this gene are found out in most tumors originating from the gastrointestinal system, urogenital system, and skin.(3) The wild-type p53 gene is involved in the differentiation, proliferation and apoptosis of cells, whereas the mutant type is considered to be the cause of atypical cell growth.(4)
However, the correlation of expression of c-erbB-2 and p53 with clinicopathologic and prognostic features is controversial.(5-10) We investigated if there is a correlation between abnormal expression of c-erbB-2 and p53 genes and the clinicopathologic features of the tumor. In addition, it was studied that the expression of two gene can be prognostic factors of gastric adenocarcinomas.
One hundred twenty-five patients who underwent curative gastric resection for gastric adenocarcinoma from January 2000 to June 2003 in St. Mary's Hospital were enrolled for this study. In this period, cancer tissues from all patients were investigated the expression of c-erbB-2 and p53. The results of staining were reported accompanying with pathologic results. We retrospectively reviewed this data. We obtained informed consent from all patients provided about supplement of tissues and immunohistochemical staining.
Curative gastric resection was performed according to treatment guideline for gastric cancer suggested by Japanese Gastric Cancer Association. After curative operation, adjuvant chemotherapy based on 5-FU was performed for patients diagnosed with over stage II according to 6th edition of American Joint Committee on Cancer (AJCC) staging system.
Cancer stage according to 6th edition of AJCC satging, tumor invasiveness, involvement of lymph nodes, histological features and the Laurén classification were evaluated by reviewing pathology reports. Recurrence and death were determined according to the follow-up database at our institution.
Tumor tissues were fixed in 10% formalin and embedded in paraffin. Immunohistochemical staining was carried out using anti-HER-2/neu (Dako, Glostrup, Denmark) as the primary antibody for c-erbB-2. After making slices using a microtome, tissue sections (4µm) were immersed in xylene solution to remove residual paraffin and hydrated in an alcohol series. Sections were boiled for 5 min to retrieve antigenicity in citrate buffer (pH 6.0) and left for 30 min at room temperature.
After exhausting endogenous peroxidase for 10 min with H2O2 in methyl alcohol, sections were washed thrice with phosphate-buffered saline (PBS). Sections were blocked for 30 min with blocking solution (Histostain™ kit, Zymed Company, San Francisco, CA, USA) at room temperature. Sections were then incubated with anti-HER-2/neu (1:200, Dako) at room temperature. After rinsing thrice with PBS, sections were incubated with biotinylated anti-mouse IgG (1:300; Zymed). After washing, sections were incubated with avidin-alkaline phosphatase for 7 min at 40℃. Sections were visualized with red chromogen at 40℃ and counterstained using the Mayer hematoxylin method. Sections were mounted and observed under light microscopy.
In general, immunohistochemical staining of p53 was done with the same staining protocol of that for c-erbB-2. Immunohistochemical staining was carried out using anti-p53 monoclonal antibody (1:100, clone DO-7; Dako) as the primary antibody. Sections were incubated for 1 h at room temperature. Sections were also visualized with red chromogen at 40℃ and counterstained with Mayer's hematoxylin.
Two pathologists independently assessed the degree of staining without clinical information of the patient. For c-erbB-2, a scoring system was applied according to location and degree of completion of staining: 0 points: staining of ≤10% was equivalent; 1 point: incomplete membrane staining of >10%; 2 points: weak-to-moderate complete staining of the membrane; and 3 points: strong complete staining of the membrane. A score of ≥2 points was classified as positive staining (Fig. 1). A degree of staining of the nucleus of >10% was classified as positive p53 staining (Fig. 2).
Statistical analysis was done with the Statistical Package for Social Science (SPSS Corporation, CA, USA) version 13.0. P<0.05 was considered significant. The correlations between expression of both proteins and clinicopathologic factors were analyzed using the chi-square test. The Kaplan-Meier method with the log-rank test was used for univariate analysis of the correlation between gene expression and survival. Factors that showed a P-value of <0.1 became candidates for multivariate analysis. Multivariate analysis for identifying the prognostic factors was done with the Cox-proportional hazard model.
The mean age of patients was 59.4±12.8 (means±SD) years. There were 86 males and 39 females. In the pathology reports, 56 patients were diagnosed with early gastric cancer, and 86 patients with advanced gastric cancer. The mean follow-up period was 48.4±10.7 months (range, 2~86 months). Twenty-four patients had recurrence and 18 patients died relating with gastric cancer. Patients with positive staining for c-erbB-2 and p53 were 24% (30/125) and 57.6% (72/125), respectively. The percentage of positive staining for both c-erbB-2 and p53 was 19.2% (24/125).
The positive staining of c-erbB-2 was significantly related to tumor invasion (P=0.022), lymph node metastasis (P=0.004) and lymphatic invasion (P=0.019). p53 staining was associated with depth of invasion (P=0.023). Other factors did not correlate with staining (Table 1).
Mean disease-free survival of 125 patients was 72.4 (65.5~75.3) months (95% confidence interval (CI)) and disease-specific overall survival was 75.2 (70.9~97.4) months (95% CI) (Fig. 3). In the univariate analysis for survival, patients with expressions of both c-erbB-2 and p53 gene showed a significantly low disease-free survival (P=0.041) (Table 2), although expression of each c-erbB-2 and p53 did not show significance. The expression of both c-erbB-2 and p53 was not predictive factor for recurrence in multivariate analysis (Table 3). In view of overall survival, immunohistochemical staining was not significant in univariate and multivariate analysis (Table 2, 3).
Subgroup analysis was carried out for 91 patients diagnosed with pathologic T2 and below. c-erbB-2 expression (P=0.025) and simultaneous expression of c-erbB-2 and p53 (P=0.004) were related to early recurrence in univariate analysis (Fig. 4). Disease-specific overall survival was also associated with expression of both c-erbB-2 and p53 (P=0.045) (Table 4). In multivariate analysis, expression of both c-erbB-2 and p53 was a predictive factor for recurrence (odds ratio (OR); 5.105, 95% CI: 1.282~20.341), but that was not significantly related with disease specific overall survival rate (Table 5).
We investigated the expression of c-erbB-2 and p53 by immunohistochemical staining in gastric cancer. Analysis of the correlation of expression of these proteins with clinicopathologic results revealed that expression of both protein was associated with aggressive pathologic features. The expression of both c-erbB-2 and p53 may be a predictive factor for recurrence, particularly in cases of relatively early stage cancer.
Recent studies have focused on predicting tumor progression by analysis of the gene expression related to carcinogenesis in gastric cancer.(11-13) These studies support the essential change of major genes for advance of gastric cancer. Attempts to develop a clinical application have been carried out based on these results. In connection with this, we planned to ascertain whether the expression of c-erbB-2 and p53 genes, major oncogenes and tumor suppressor genes, are related to clinicopathologic features and the prognosis.
The c-erbB-2 oncogene is critically related with epidermal growth factor (EGF) receptor. The level of protein expressed by c-erbB-2 gene is increased in various adenocarcinoma tissue such as breast, ovary, cervix and lung; this expression is associated with aggressive clinical features and poor prognosis.(14,15) In gastric cancer, several studies reported that the expression rate of c-erbB-2 varied from 9% to 45%;(5,16,17) our result was 24%. This multiplicity of the rate of c-erbB-2 expression is thought to be due to differences in sample size. Other reports stated that c-erbB-2 expression is differed according to the histological type of gastric cancer, but correlation with prognosis is controversial.(10,18) In this study, c-erbB-2 expression was related to depth of invasion of tumor, lymph node metastasis and lymphatic invasion presenting aggressive features of the tumor. According to in univariate and multivariate analysis, this study did not show that patients with c-erbB-2 expressed cancer had a significantly shorter disease-free or disease-specific overall survival than that of patients who did not have c-erbB-2-expressed cancer. c-erbB-2 positive patients with p53 expression had significantly lower disease-free survival in only univariate analysis. Particularly, in the analysis of patients who did not have serosa-exposed tumors, positive expression of c-erbB-2 and p53 was correlated with shorter disease-free and overall survival in univariate analysis, and could be a significant factor for predicting cancer recurrence in multivariate analysis. Expression of two genes could be one of the prognostic factors only for patients with relatively early stage tumors. This may be because the prognosis of patients with more advanced gastric cancer, serosa exposed, may be affected by other factors as well as the role in carcinogenesis of these genes.
The major functions of p53 protein are regulation of the cell cycle and apoptosis, and repair of DNA damage. Functional abnormality of p53 is known to be caused by mutation of the p53 gene, including loss of heterozygosity (LOH) and DNA methylation,(19) which can affect the biological behavior of the tumor and therefore prognosis. Several studies have been conducted to find the correlation of abnormal expression of p53 with prognosis in cancer of the colon,(20) and breast,(7) but the results were inconclusive. For gastric cancer, p53 expression was 40~60% and was related to disease progression, and the degree of cellular division,(6-8,21) but studies reporting the correlation of p53 expression with prognosis are lacking. In the present study, p53 expression was 57.6% and tumor-expressed p53 have correlated with depth of tumor invasion, but a relationship with prognosis was not evident.
In the present study, examination of c-erbB-2 or p53 is sensible because these two genes are located on the same chromosome, and carcinogenesis by multistep mutation of genes could occur in gastric cancer. Chang et al.(22) reported that high expression of p53 was strictly related to c-erbB-2 expression in breast cancer. In gastric cancer, a report in which expression of p53 and c-erbB-2 was evaluated showed correlation between these two genes.(23) In the present study, of 30 tumors expressing c-erbB-2, 24 tumors (80%) also showed expression of p53. Statistically significant results were obtained for patients who had simultaneous expression of c-erbB-2 and p53: they had a poorer prognosis than that of the others. We therefore hypothesize that mutations of these two genes has a part in the carcinogenesis of gastric cancer.
New agents targeting the molecules correlated with tumor carcinogenesis have been developed. Agents focused on the c-erbB-2 and p53 genes, as representative oncogenes and tumor suppressor genes, have been studied and transfer to the clinical setting attempted. The current experimental approach targeting p53 aims to induce apoptosis or to prevent destruction of normal cells by chemotherapy.(24) Bykov et al.(25) reported that agents targeting a mutant p53 gene could be effective without apparent toxic side effects, although these results were limited to an in-vivo pilot study. When c-erbB-2 has been used as a targeting molecule, agents such as monoclonal antibodies that can inhibit appropriate ligands combining with EGF receptors associated with c-erbB-2 expression have been developed.(26) In gastric cancer, there is no evidence about the clinical benefit of agents targeting specific molecules, and several clinical trials enrolling patients with nonoperable gastric cancer are ongoing.(27) If clinical benefit can be shown in these trials, the concept of using targeting agents can be expanded into the adjuvant setting to lower recurrence after curative resection.
Adjuvant chemotherapy for gastric cancer has focused on oral fluorouracil agents. A clinical trial showed that adjuvant treatment with uracil-tegafur for patients with non-serosa-exposed tumor resected curatively could reduce recurrence and enhance survival.(28) The result of that trial showed that the five-year survival of these patients was 86%, and that 33% of patients complained that chemotherapy induced toxicity of grade >3 grade, even though the study enrolled patients with less advanced cancer. To improve prognosis and reduce chemotherapy-related toxicity, we hypothesize that these patients should be have a new adjuvant strategy according to markers expressed in the tumor and which correlated with prognosis. In the present study, simultaneous expression of c-erbB-2 and p53 was a significant factor for prediction of recurrence and survival: using agents targetting those two molecules may merit further investigation.
Figures and Tables
Fig. 1
Immunohistochemical staining of c-erbB-2. (A) No expression (0 points), (B) 1~10% expression (1 point), (C) 10~50% expression (2 points), (D) >50% expression (3 points). Scale bar=50µm
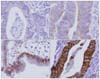
Fig. 2
Immunohistochemical staining of p53. (A) <10% expression, (B) >10% expression. Scale bar=50µm.

Fig. 3
Disease-free survival (A) and overall survival (B) according to expression of both c-erbB-2 and p53 in all patients, P was calculated by the log rank test.

Fig. 4
Disease-free survival (A) and overall survival (B) according to expression of both c-erbB-2 and p53 in patients with tumors not exposed to serosa, P was calculated by the log rank test.

Table 2
Comparison of disease-free and overall survival according to clininopathologic factors and results of immunohistochemical staining (n=125)

References
1. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993. 54:594–606.
2. Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984. 312:513–516.
3. Hernandez-Boussard T, Rodriguez-Tome P, Montesano R, Hainaut P. International Agency for Research on Cancer. IARC p53 mutation database: a relational database to compile and analyze p53 mutations in human tumors and cell lines. Hum Mutat. 1999. 14:1–8.
4. Matozaki T, Sakamoto C, Suzuki T, Matsuda K, Uchida T, Nakano O, et al. p53 gene mutations in human gastric cancer: wild-type p53 but not mutant p53 suppresses growth of human gastric cancer cells. Cancer Res. 1992. 52:4335–4341.
5. Hilton DA, West KP. c-erbB-2 oncogene product expression and prognosis in gastric carcinoma. J Clin Pathol. 1992. 45:454–456.
6. Ichiyoshi Y, Oiwa H, Tomisaki S, Sakaguchi Y, Ohno S, Maehara Y, et al. Overexpression of p53 is associated with growth pattern and prognosis in advanced gastric cancer. Hepatogastroenterology. 1997. 44:546–553.
7. Ostrowski JL, Sawan A, Henry L, Wright C, Henry JA, Hennessy C, et al. p53 expression in human breast cancer related to survival and prognostic factors: an immunohistochemical study. J Pathol. 1991. 164:75–81.
8. Starzynska T, Bromley M, Ghosh A, Stern PL. Prognostic significance of p53 overexpression in gastric and colorectal carcinoma. Br J Cancer. 1992. 66:558–562.
9. Yonemura Y, Ninomiya I, Ohoyama S, Kimura H, Yamaguchi A, Fushida S, et al. Expression of c-erbB-2 oncoprotein in gastric carcinoma. Immunoreactivity for c-erbB-2 protein is an independent indicator of poor short-term prognosis in patients with gastric carcinoma. Cancer. 1991. 67:2914–2918.
10. Yonemura Y, Ninomiya I, Yamaguchi A, Fushida S, Kimura H, Ohoyama S, et al. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res. 1991. 51:1034–1038.
11. Becker KF, Keller G, Hoefler H. The use of molecular biology in diagnosis and prognosis of gastric cancer. Surg Oncol. 2000. 9:5–11.
12. Tamura G. Genetic and epigenetic alterations of tumor suppressor and tumor-related genes in gastric cancer. Histol Histopathol. 2002. 17:323–329.
13. Tamura G. Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World J Gastroenterol. 2006. 12:192–198.
14. Christensen JG, Schreck RE, Chan E, Wang X, Yang C, Liu L, et al. High levels of HER-2 expression alter the ability of epidermal growth factor receptor (EGFR) family tyrosine kinase inhibitors to inhibit EGFR phosphorylation in vivo. Clin Cancer Res. 2001. 7:4230–4238.
15. Danielsen AJ, Maihle NJ. Ligand-independent oncogenic transformation by the EGF receptor requires kinase domain catalytic activity. Exp Cell Res. 2002. 275:9–16.
16. Allgayer H, Babic R, Gruetzner KU, Tarabichi A, Schildberg FW, Heiss MM. c-erbB-2 is of independent prognostic relevance in gastric cancer and is associated with the expression of tumor-associated protease systems. J Clin Oncol. 2000. 18:2201–2209.
17. Jain S, Filipe MI, Gullick WJ, Linehan J, Morris RW. c-erbB-2 proto-oncogene expression and its relationship to survival in gastric carcinoma: an immunohistochemical study on archival material. Int J Cancer. 1991. 48:668–671.
18. Tateishi M, Toda T, Minamisono Y, Nagasaki S. Clinicopathological significance of c-erbB-2 protein expression in human gastric carcinoma. J Surg Oncol. 1992. 49:209–212.
19. Hollstein M, Rice K, Greenblatt MS, Soussi T, Fuchs R, Sorlie T, et al. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994. 22:3551–3555.
20. Scott N, Sagar P, Stewart J, Blair GE, Dixon MF, Quirke P. p53 in colorectal cancer: clinicopathological correlation and prognostic significance. Br J Cancer. 1991. 63:317–319.
21. Ioachim E, Goussia A, Stefanou D, Agnantis NJ. Expression of p53 protein in gastric cancer: an immunohistochemical study with correlation to proliferative activity. Anticancer Res. 1997. 17:513–517.
22. Chang K, Ding I, Kern FG, Willingham MC. Immunohistochemical analysis of p53 and HER-2/neu proteins in human tumors. J Histochem Cytochem. 1991. 39:1281–1287.
23. Sasano H, Date F, Imatani A, Asaki S, Nagura H. Double immunostaining for c-erbB-2 and p53 in human stomach cancer cells. Hum Pathol. 1993. 24:584–589.
24. Bouchet BP, de Fromentel CC, Puisieux A, Galmarini CM. p53 as a target for anti-cancer drug development. Crit Rev Oncol Hematol. 2006. 58:190–207.
25. Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002. 8:282–288.
26. Baxevanis CN, Sotiropoulou PA, Sotiriadou NN, Papamichail M. Immunobiology of HER-2/neu oncoprotein and its potential application in cancer immunotherapy. Cancer Immunol Immunother. 2004. 53:166–175.
27. Ohtsu A. Chemotherapy for metastatic gastric cancer: past, present, and future. J Gastroenterol. 2008. 43:256–264.
28. Nakajima T, Kinoshita T, Nashimoto A, Sairenji M, Yamaguchi T, Sakamoto J, et al. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br J Surg. 2007. 94:1468–1476.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download