Abstract
Purpose
Iron plays an important role in the process of oxidizing Low Density Lipoprotein (LDL) in the arterial wall during the development of atherosclerosis, but the role of iron during the development of intimal hyperplasia has not been confirmed. Therefore, we evaluated the relationship of serum ferritin, serum cholesterol and intimal hyperplasia.
Methods
Forty rats were divided into four groups according to diet. Group I was the normocholesterol and normoferritin group, group II was the hypercholesterol and normoferritin group, group III was the hypercholesterol and hypoferritin group, and group IV was the hypercholesterol and hyperferritin group. At the sixth week, we induced clamping injury at the left common carotid artery of each rat. At the end of the eighth week, we obtained tissue of the left common carotid artery from each rat, and we performed staining. After that, we evaluated differences of the intima to media ratio (IMR) of arterial walls according to groups.
Atherosclerosis is a multifactorial disease. Oxidation, and particularly modification of low density lipoproteins (LDL) within the artery wall and their subsequent unregulated uptake by macrophages has been postulated to be important in disease development, and metal ions such as iron and copper have an important role in this process.(1) Several reports that have focused on the relationship of iron and atherosclerotic disease have currently been reported. Sullivan showed that premenopausal women had a lower risk than aged-matched men in developing coronary artery disease, and menopausal women had a higher risk of developing coronary artery disease than age-matched men: this suggests the iron-heart hypothesis in the development of atherosclerotic diseases.(2) Haidari et al.(3) reported on the relationship of serum ferritin and cororary artery disease in male patients younger than fifty years. Ramakrishna et al.(4) reported on the relationship of iron and athereosclerosis in young people. But, there is currently controversy about the relationship of iron, atherosclerotic disease, and intimal hyperplasia. So, to identify the relationship of iron, atherosclerotic disease, and intimal hyperplasia, we herein attempt to evaluate the relationship of serum ferritin as the most accurate marker of the body's storage of iron,(5) and the intima to media ratio (IMR) of carotid artery induced intimal hyperplasia and atherosclerotic disease in a rat model. This research was approved by the IACUC in the School of Medicine of the Catholic University of Korea.
This experiment was conducted on forty rats (Sprague-Dawley rats) that were eight weeks old. We divided the forty rats into four groups of 10 rats each. The rats in group I were given a control diet and control water for eight weeks, and the rats in group II were given a hypercholesterol diet and control water for eight weeks.(6) The rats in group III were given a hypercholesterol diet and water mixed with an iron-chelating agent (Exjade) 30 mg/kg/day (Novartis, Basel, Swiss) for eight weeks. The rats in group IV were given a hypercholesterol diet with control water for eight weeks, and they were injected intraperitoneally with an iron-containing agent (Venoferrum) 50 mg/rat/week (Choongwae Pharma Corporation, Seoul, Korea) for six weeks during the study.(7) The control diet was the Low Fat, Low Cholesterol Control Diet (D12337) (Research Diets, New Brunswick, NJ, USA), and the hypercholesterol diet was Paigen's Atherogenic Rodent diet (D12336) (Research Diets, New Brunswick, NJ, USA).
This experiment was run for eight weeks. At the sixth week, all the rats underwent a "clamping injury" by one-click clamping on the left common carotid arteries by using a Mosquito clamp (the same type of clamp was used on all rats) for thirty seconds under inhalation anesthesia (2% Isoflurane). After releasing the clamp, we observed the blood flow in the left common catrotid arteries by Doppler ultrasonography. At end of the eighth week, we obtained the left carotid artery tissue and blood (3 ml) from each rat with the rats under inhalation anesthesia (2% Isoflurane). After that, we euthanized the rats with carbon dioxide (CO2) according to the guidelines of our institute. We measured the body weights of all the rats at the first day and at the end of the eighth week. We then evaluated the Iron, ferritin, Total Iron Binding Capacity (TIBC), Total Cholesterol (TC), Triglyceride (TG), High Density Lipoprotein (HDL), and Low Density Lipoprotein (LDL) in the blood from each rat, and performed Hematoxylin & Eosin (H&E) staining, Masson-Trichrome staining, and immunohistochemistry with Alpha Smooth Muscle Actin (α-SMA) antibody (Amersham Pharmacia Biotech, Piscataway, NJ, USA) on the sections cut after 10% formalin fixation of the carotid artery tissues of each rat and embedded them in a paraffin block.(8) We then measured the IMRs of the left carotid arteries using a computer program (TDI Scope Eye, Techsan, Korea).
The carotid artery tissues were cut into sagittal slices of 1~2µm thickness and post-fixed overnight in a periodate-lysine-paraformaldehyde (PLP) solution at 4℃. A portion of the PLP-fixed carotid artery tissue was embedded in wax for trichrome staining. After dewaxing, 4µm sections were processed and stained with Masson's trichrome.
For detection of α-SMA in tissues, the tissue sections were incubated with 0.5% TritonX-100 (Sigma-Aldrich Co, St. Louis, MO, USA) in 0.01 M phosphate-buffered saline (PBS) and then blocked with normal donkey serum. Subsequently, the tissue sections were incubated overnight at 4℃ with primary antibodies against α-SMA. The tissue sections were rinsed in PBS and incubated in peroxidase-conjugated donkey anti-mouse or rabbit antimouse IgG (Amersham Pharmacia Biotech, Piscataway, NJ, USA). After being rinsed with a Tris-buffered saline (TBS) (Sigma-Aldrich Co, St. Louis, MO, USA), the tissue sections were incubated with a mixture of 0.05% 3,3-diaminobenzidine until a brown colour was visible, washed with tris-buffered saline (TBS), counterstained with haematoxylin, and examined under light microscopy. The number of α-SMA-positive cells was quantified in a 0.5 mm2 area of rat carotid artery using a computer program (TDI Scope Eye). The 20 fields per section were assessed. All morphometric measurements and gradings were performed in a blinded manner by two examiners.
Five rats were excluded due to massive bleeding after the clamping injury in three rats in group IV at the sixth week, and the missing of arterial tissues in two rats in group IV. So, ultimately, thirty-five rats were included in the final results. We performed H&E staining on immediately harvested carotid artery tissues following the clamping injury from expired rats due to massive bleeding after the clamping injury to ascertain proof of the clamping injury-induced early endothelial denudation (Fig. 1). Over an eight-week period, the increased body weights were 148.1±36.1 g (group I), 171.5±34.2 g (group II), 241.2±29.9 g (group III), and 173.4±71.6 g (group IV). In group III, the body weights were significantly increased. The body weights in group IV were less increased than those in group III (Fig. 2). Between group I and group II, TC, LDL, and TG were significantly more increased in group II (P<0.05) (Fig. 2). So, group I and group II showed significant differences with respect to the lipid profiles. Among the groups that received a hypercholesterol diet (group II~IV), TC and LDL were significantly increased from group II to group IV, respectively (P<0.05) (Fig. 2). Between group I and group II, there was a significant difference in the concentration of serum iron (183.2±29.7µg/dl vs 154.1±30.0µg/dl) (P<0.05), and there were no significant differences in the concentration of TIBC (527.7±97.2µg/dl vs 507.1±126.8µg/dl) (P=0.688) and ferritin (54.6±16.6 µg/L vs 46.4±7.3µg/L) (P=0.344) (Fig. 2). Among the groups that received a hypercholesterol diet (group II~IV), there was an insignificant difference in the concentration of serum iron between group II (154.1±30.0µg/dl) and group III (137.4±27.6µg/dl) (P=0.212), but the concentration of serum iron between group II (154.1±30.0µg/dl) and group IV (205±80.7µg/dl) were significantly different (P=0.019). The TIBC among groups II, III and IV were significantly different (507.1±126.8µg/dl (group II), 844.6±84.8µg/dl (group III) and 801.9±82.7µg/dl (group IV) (P<0.05). But serum ferritin as an adequate index of tissue iron stores(5) were significantly different between group II and group III and between group II and group IV, respectively (46.4±7.3µg/L (group II) and 35.7±7.4µg/L (group III) (P=0.004) and 46.4±7.3µg/L (group II) and 264.4±136.8µg/L (group IV) (P<0.001)) (Fig. 2). So, the groups that ate a hypercholesterol diet (group II~IV) were significantly different with respect to the concentration of iron. By microscopic findings, the arterial thickness and cellularity in group II were increased more than those in group I on the H&E staining (Fig. 3, 4). Among the hypercholesterol groups (groups II~IV), the arterial thickness and cellularity in group II were increased more than those in group III, and those in group IV were more increased than those in group II on the H&E staining (Fig. 3, 4). The collagen materials of the matrix in group IV were more increased than those in other groups on the Masson-Trichrome staining (Fig. 5). The migration and proliferation of smooth muscle cells in group I were somewhat expressed as was seen on the immunohistochemistry (Fig. 6). The migration and proliferation of smooth muscle cells in group II were more expressed than those in group I as was seen on the immunohistochemistry (Fig. 6). Among the hypercholesterol groups (groups II~IV), the migration and proliferation of smooth muscle cells in group II were more expressed than those in group III, and those in group IV were more expressed than those in group II on the immunohistochemistry with α-SMA antibody (Fig. 6). To the objective analysis, we measured the arterial wall thickness, the intimal thickness and the IMR of the left carotid arteries using a computer program (TDI Scope Eye, Techsan, Korea). The arterial wall thicknesses were significantly increased from group I (108.8±9.0µm) to group IV (137.6±3.9µm) (P<0.05) (Fig. 7). The intimal thicknesses in group II (72.9±6.4µm) were significantly more increased than those in group I (58.0±3.9µm) (P<0.001) (Fig. 7). Among the hypercholesterol groups (groups II~IV), the intimal thicknesses in group III (67.0±3.1µm) were less increased than those in group II (72.9±6.4µm) (P=0.023), and the intimal thicknesses in group IV (92.6±2.9µm) were more increased than those in group II (72.9±6.4µm) (P=0.002) (Fig. 7). The IMRs in group II (1.57±0.22µm) were significantly more increased than those in group I (1.15±0.06µm) (P<0.001) (Fig. 7). Among the hypercholesterol groups (groups II~IV), the IMRs in group III (1.18±0.03µm) increased less than those in group II (1.57±0.22µm) (P<0.001), and the IMRs in group IV (2.06±0.11µm) were more increased than those in group II (1.57±0.22µm) (P=0.007) (Fig. 7).
A putative role of iron as a contributor to the development of atherosclerosis has been discussed.(2) Sullivan(2) formulated the iron-heart hypothesis of atherosclerotic cardiovascular disease to explain the age-related increased risk of myocardial infarction (MI) in women following menopause, and emphasized the differential coronary risk between males and females before the fifth decade of life. Haidari et al.(3) reported a significant relationship between serum ferritin levels and the risk of coronary artery disease in male patients who were younger than 50 years. Ramakrishna et al.(4) reported evidence that was consistent with the contribution of iron to atherosclerosis, but at a relatively earlier age. The proatherogenic properties of iron have been thought to be proportionally related with its ability to generate reactive oxygen species, to oxidize lipoproteins, and to activate platelets.(9-11) This hypothesis is in agreement with several epidemiological studies that observed an association between measures of the body's iron stores and myocardial infarction, as well as with the progression of carotid atherosclerosis.(12-14) Further, the Kuopio study(12) and the Bruneck study(14) have described a synergistic interaction between the serum ferritin levels and LDL cholesterol as to their relationship with carotid atherosclerosis. But, the role of iron during the development of intimal hyperplasia has been not confirmed.
In the literature,(15) the relationship between the serum ferritin levels and carotid atherosclerosis might have been confounded by inflammation and mild liver disease, and especially among the subjects with metabolic syndrome and diabetes, which are conditions that are strongly correlated with atherosclerosis. Although we did not exclude the subjects with malignancies and manifest liver disease, the residual confounding by such factors cannot be ruled out. This is one of our study's limitations.
In our experiment, intimal hyperplasia of the carotid artery was more significantly increased in the hypercholesterol group than that in the control group, and among the hypercholesterol groups, intimal hyperplasia of the carotid artery was significantly proportionally related with serum ferritin. That is, the higher the serum ferritin level, the more increased the intimal hyperplasia of the carotid artery. The serum ferritin levels have been proposed in the literature as an adequate index of tissue iron stores.(5) Thus, we used the serum ferritin levels as an index of the iron stores.
After mechanical injury/stimulation by balloon injury or clamping injury, the cells in the arterial wall change their phenotype to the activated state, and attachment of platelets to the injured surface area and their activation/aggregation occur almost immediately, and the migration of leukocytes follows.(16,17) The platelets and leukocytes distributed in the injured blood vessel release reactive oxygen species and this enhances the oxidative stress on the vessel wall. These cells also release cytokines that induce the migration and proliferation of Vascular Smooth Muscle Cells (VSMCs).(16) In addition to being expressed in endothelial cells, oxidized LDL receptor (LOX-1) is expressed in smooth muscle cells, macrophages, and platelets, and LOX-1 is involved in the process of intimal hyperplasia after intimal injury.(18-20) After clamping injuries to the carotid arteries of the rats, we obtained the carotid arterial tissues. We did formalin fixation and embedded the tissues into paraffin blocks. After cutting sections, we performed staining with 3 different stains. The first one was H&E stain to evaluate the various cellular densities and the IMRs. The second one was Masson-Trichrome stain to assess the collagen content within the matrix. The last one was immunohistochemistry with α-SMA antibody to evaluate the aspects of vascular myofibroblasts and to assess migration and proliferation of smooth muscle cells.(8) Between the control group and the hypercholesterol groups, the measured IMRs increased in the hypercholesterol groups more than that in the control group. Among the hypercholesterol groups, the measured IMRs were significantly, proportionally related with the serum ferritin levels. That is, the higher the serum ferritin level, the higher the IMR.
These results have been similarly reported by several animal experiments.(16,21) Hinagata et al.(16) have reported that the oxidized LDL receptor (LOX-1) expressed in endothelial cells and smooth muscle cells was involved in intimal hyperplasia in a rat model of balloon injury, and the administration of an anti-LOX-1 antibody suppressed the neointimal thickening that occurred after balloon injury. Day et al.(21) have reported that moderate iron loading markedly accelerated thrombus formation after arterial injury, it increased vascular oxidative stress and it impaired vasoreactivity. The iron-induced vascular dysfunction might contribute to the increased incidence of ischemic cardiovascular events which are associated with chronic iron overload.(21)
Similar results from research on humans have been also reported.(12,14,15) In the Kupio study,(12) it was reported that the concentrations of iron-stores in Eastern people were proportionally related with the risk of myocardial infarction. In the Bruneck study,(14) it was reported that body iron levels were proportionally related with the risk of carotid atherosclerosis. Wolff et al.(15) reported that there was an independent relationship between serum ferritin levels and carotid atherosclerosis, and this relationship was strengthened by a synergistic association between ferritin and LDL cholesterol.
Molecular studies and a recent proteomic study of human tissues also support the iron hypothesis. Smith et al.(22) reported that iron deposition was found to be high in atherosclerotic lesions. Pang et al.(23) reported that Northern blot analysis showed that the expression of H- and L-ferritin mRNAs were higher in human and rabbit atherosclerotic aortas than those in normal human and rabbit aortas. Yuan et al.(24) showed that the binding of macrophages to oxidized LDL was significantly enhanced following erythrophagocytosis, and LDL oxidation was inhibited by desferrioxamine, which is an iron chelator.
Despite these results, the relationship between serum ferritin levels, intimal hyperplasia, and atherosclerotic disease has not been completely confirmed. We think that this is because several reports have shown conflicting results(25) and the prospective studies and molecular studies in humans have been insufficient.
In conclusion, we suggest that the iron concentration (serum ferritin) and cholesterol levels are proportionally related with intimal hyperplasia after arterial injury. We think that this relationship should be confirmed by more prospective studies and molecular studies. Also, by confirming the obvious role of iron in the pathogenesis of intimal hyperplasia, we hope that we can prevent intimal hyperplasia by lowering iron and cholesterol levels after arterial anastomosis in the future.
Figures and Tables
Fig. 1
H&E stains on immediately harvested carotid artery tissues following the clamping injury shows the clamping injury-induced early endothelial denudation. (A) shows the endothelial denudation of common carotid arteries of rats in H&E stain (×100), and (B~D) show the endothelial denudation of common carotid arteries of rats in H&E stain (×200). The arrows indicate the endothelial denudation of common carotid arteries.
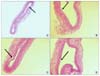
Fig. 2
The laboratory findings of the rats show differences according to the groups. (A) shows the difference of the increase of body weight, (B) shows that of the Total Cholesterol (TC), (C) shows that of the Triglyceride (TG), (D) shows that of the High Density Lipoprotein (HDL), (E) shows that of the Low Density Lipoprotein (LDL), (F) shows that of the Ferritin, (G) shows that of the Iron, and (H) shows that of the Total Iron Binding Capacity (TIBC). The * means P<0.05.

Fig. 3
H&E stains (×100) of the common carotid arteries of the rats show differences according to the groups. (A) shows the common carotid arteries of the group I rats, (B) shows the common carotid arteries of the group II rats, (C) shows the common carotid arteries of the group III rats, and (D) shows the common carotid arteries of the group IV rats.
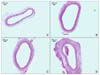
Fig. 4
H&E stains (×200) of the common carotid arteries of the rats show differences according to the groups. (A) shows the common carotid arteries of the group I rats, (B) shows the common carotid arteries of the group II rats, (C) shows the common carotid arteries of the group III rats, and (D) shows the common carotid arteries of the group IV rats. The arrows indicate the intimal thickness of each group.
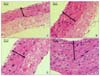
Fig. 5
Masson-Trichrome stains (×200) of the common carotid arteries of the rats show differences according to the groups. (A) shows the common carotid arteries of the group I rats, (B) shows the common carotid arteries of the group II rats, (C) shows the common carotid arteries of the group III rats, and (D) shows the common carotid arteries of the group IV rats. The arrow indicates the collagen materials of the matrix (blue color) of each group.
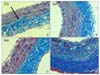
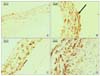
Fig. 6
The immunohistochemistry using anti-α-SMA antibody (×200) of the common carotid arteries of the rats show differences according to the groups. (A) shows the common carotid arteries of the group I rats, (B) shows the common carotid arteries of the group II rats, (C) shows the common carotid arteries of the group III rats, and (D) shows the common carotid arteries of the group IV rats. The arrow indicates the α-SMA-positive cells of each group.
References
1. Heinecke JW. Oxidants and antioxidants in the pathogenesis of atherosclerosis: implications for the oxidized low density lipoprotein hypothesis. Atherosclerosis. 1998. 141:1–15.
2. Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981. 1:1293–1294.
3. Haidari M, Javadi E, Sanati A, Hajilooi M, Ghanbili J. Association of increased ferritin with premature coronary stenosis in men. Clin Chem. 2001. 47:1666–1672.
4. Ramakrishna G, Rooke TW, Cooper LT. Iron and peripheral arterial disease: revisiting the iron hypothesis in a different light. Vasc Med. 2003. 8:203–210.
5. You SA, Wang Q. Ferritin in atherosclerosis. Clin Chim Acta. 2005. 357:1–16.
6. Chiang MT, Chen YC, Huang AL. Plasma lipoprotein cholesterol levels in rats fed a diet enriched in cholesterol and cholic acid. Int J Vitam Nutr Res. 1998. 68:328–334.
7. Holbein BE. Iron-controlled infection with Neisseria meningitidis in mice. Infect Immun. 1980. 29:886–891.
8. Han JG, Xu HM, Song WL, Jin ML, Gao JS, Wang ZJ, et al. Histologic analysis of acellular dermal matrix in the treatment of anal fistula in an animal model. J Am Coll Surg. 2009. 208:1099–1106.
9. Yuan XM, Brunk UT, Olsson AG. Effects of iron- and hemoglobin-loaded human monocyte-derived macrophages on oxidation and uptake of LDL. Arterioscler Thromb Vasc Biol. 1995. 15:1345–1351.
10. Lamb DJ, Leake DS. Iron released from transferring at acidic pH can catalyse the oxidation of low density lipoprotein. FEBS Lett. 1994. 352:15–18.
11. Pratico D, Pasin M, Barry OP, Ghiselli A, Sabatino G, Iuliano L, et al. Iron-dependent human platelet activation and hydroxyl radical formation: involvement of protein kinase C. Circulation. 1999. 99:3118–3124.
12. Salonen JT, Nyyssonen K, Korpela H, Tuomilehto J, Seppanen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992. 86:803–811.
13. Magnusson MK, Sigfusson N, Sigvaldason H, Johannesson GM, Magnusson S, Thorgeirsson G. Low iron-binding capacity as a risk factor for myocardial infarction. Circulation. 1994. 89:102–108.
14. Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997. 96:3300–3307.
15. Wolff B, Völzke H, Lüdemann J, Robinson D, Vogelgesang D, Staudt A, et al. Association between high serum ferritin levels and carotid atherosclerosis in the study of health in Pomerania (SHIP). Stroke. 2004. 35:453–457.
16. Hinagata J, Kakutani M, Fujii T, Naruko T, Inoue N, Fujita Y, et al. Oxidized LDL receptor LOX-1 is involved in neointimal hyperplasia after balloon arterial injury in a rat model. Cardiovasc Res. 2006. 69:263–271.
17. Bennett MR, O'Sullivan M. Mechanisms of angioplasty and stent restenosis: implications for design of rational therapy. Pharmacol Ther. 2001. 91:149–146.
18. Aoyama T, Chen M, Fujiwara H, Masaki T, Sawamura T. LOX-1 mediates lysophosphatidylcholin-induces oxidized LDL uptake in smooth muscle cells. FEBS Lett. 2000. 467:217–220.
19. Moriwaki H, Kume N, Kataoka H, Murase T, Nishi E, Sawamura T, et al. Expression of lectin-like oxidized low density lipoprotein receptor-1 in human and murine macrophages: upregulated expression by TNF-alpha. FEBS Lett. 1998. 440:29–32.
20. Chen M, Kakutani M, Naruko T, Ueda M, Narumiya S, Masaki T, et al. Activation-dependent surface expression of LOX-1 in human platelets. Biochem Biophys Res Commun. 2001. 282:153–158.
21. Day SM, Duquaine D, Mundada LV, Menon RG, Khan BV, Rajagopalan S, et al. Chronic iron administration increases vascular oxidative stress and accelerates arterial thrombosis. Circulation. 2003. 107:2601–2606.
22. Smith C, Mitchinson MJ, Aruoma OI, Halliwell B. Stimulation of lipid peroxidation and hydroxyl-radical generation by the contents of human atherosclerotic lesions. Biochem J. 1992. 286:901–905.
23. Pang JH, Jiang MJ, Chen YL, Wang FW, Wang DL, Chu SH, et al. Increased ferritin gene expression in atherosclerotic lesions. J Clin Invest. 1996. 97:2204–2212.
24. Yuan XM, Anders WL, Olsson AG, Brunk UT. Iron in human atheroma and LDL oxidation by macrophages following erythrophagocytosis. Atherosclerosis. 1996. 124:61–73.
25. Turbino-Ribeiro SM, Silva ME, Chianca DA Jr, De Paula H, Cardoso LM, Colombari E, et al. Iron overload in hypercholesterolemic rats affects iron homeostasis and serum lipids but not blood pressure. J Nutr. 2003. 133:15–20.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download