Abstract
Purpose
The purpose of this study is to identify useful clinicopathologic factors for the prediction of lymph node metastasis in submucosally invasive colorectal carcinoma.
Methods
A total of fifty-four cases of colorectal carcinomas with submucosal invasion were included. The patients underwent curative resection with en bloc lymph node dissection. Clinical features such as age, gender, tumor size and tumor location were reviewed. Histopathologic examinations for tumor growth type, differentiation, depth of tumor invasion, lymphovascular invasion, neural invasion, tumor budding and peritumoral inflammation were performed. The expression of E-cadherin, β-catenin, Smad4, p53 and Ki-67 were examined by immunohistochemistry. The correlation between the clinicopathologic factors and lymph node metastasis was evaluated.
Results
From the 54 patients with submucosally invasivecolorectal carcinoma, lymph node metastasis was identified in 13 cases (24.1%). The incidence of lymph node metastasis was significantly higher in cases positive for lymphovascular invasion (55.6% vs. 17.8%, P=0.028) and positive for tumor budding (47.4% vs. 11.45%, P=0.006). Cases negative for Smad4 had a higher tendency for incidence of lymph node metastasis (28.6% vs. 15.8%, P=0.341). Other clinicopathologic and immunohistochemical features were irrelevant to the lymph node status. In multivariate analysis, only tumor budding was an independent predictor of lymph node metastasis (P=0.051).
Conclusion
Lymphovascular invasion and tumor budding were predictive factors of lymph node metastasis in submucosally invasive colorectal carcinoma. The incidence of lymph node metastasis of submucosally invasive colorectal carcinoma was not low. Careful selection for avoiding surgery in submuocally invasive colorectal carcinoma should be considered.
Early colorectal carcinoma includes intramucosal carcinoma and submucosally invasive carcinoma of the colon and rectum, regardless of the presence of lymph node metastasis.(1) With advancement of endoscopic instruments and techniques, some of the early colorectal carcinomas can be safely and completely removed with endoscopic resection.(2,3) Endoscopic resection of intramucosal colorectal carcinoma is accepted as a curative therapy, because there is no risk of lymph node metastasis.(4)
Of the cases with submucosally invasive colorectal carcinoma, the incidence of lymph node metastasis has ranged from 8 to 14 percent.(5-9) Therefore, if the specimen from the endoscopic resection reveals a submucosally invasive colorectal carcinoma, an additional curative surgery with lymph node dissection is necessary. If lymph node metastasis is predictable in cases with submucosally invasive colorectal carcinoma, an additional curative surgery can selectively be avoided for cases without risk factors for lymph node metastasis. Thus, it is important to predict lymph node metastasis in submucosally invasive colorectal carcinoma so that the most appropriate treatment modality can be chosen.
Several studies have showed some predictable factors of lymph node metastasis in cases with submucosally invasive colorectal carcinoma, such as tumor differentiation, depth of tumor invasion into the submucosal layer, lymphovascular invasion, tumor budding and inflammation around the cancer.(8-10) There are many molecular markers to predict lymph node metastasis in submucosally invasive colorectal adenocarcinoma, including E-cadherin, β-catenin. Smad4, p53 and Ki-67.(11-16) However, their predictive value for lymph node metastasis in submucosally invasive colorectal carcinoma are complicated and are still up for debate.
This study was done to evaluate the significance of some clinicopathologic factors and immunohistochemical molecular markers for the prediction of lymph node metastasis in cases with submucosally invasive colorectal carcinoma.
Colorectal tissue specimens were obtained from 54 cases which had undergone curative resection for submucosally invasive colorectal carcinoma at Pusan National University Hospital and Kosin University Gospel Hospital from January 2003 to December 2007. Curative resection was defined as the removal of gross carcinoma with tumor-negative surgical margins and the en bloc resection of regional lymph nodes. There was no case received preoperative chemoradiation for colorectal carcinoma. All cases were free of distant metastasis and synchronous colorectal carcinoma. There were no cases with familial adenomatous polyposis and inflammatory bowel disease. In all specimens, tumor cells extended through the muscularis mucosa into the submucosa but did not invade the muscularis propria. Cases with less than three sampled lymph nodes were excluded.
The clinical data, such as age, gender, tumor size and tumor location in the colon and rectum were reviewed retrospectively.
The resected specimens were immediately fixed in 10% buffered formalin and the tumors were cut into step-wise sections and embedded in paraffin and stained with hematoxylin and eosin.
The lesions were classified as either polypoid growth (PG) type carcinoma or nonpolypoid growth (NPG) type carcinoma according to the classification proposed by Shimoda et al.(17) PG type-tumors show protrusions due to intramucosal proliferation of the tumor and include pedunculated, sessile and broad based proliferative lesions. NPG type-carcinomas have no intramucosal protruberant growth and exhibit sessile, flat or ulcerated growth pattern. Histologic type and grade were determined according to the World Health Organization criteria.(18)
The depth of submucosal invasion was measured as the vertical distance from the baseline, defined as the horizontal lines between the identified muscularis mucosa, to the deepest portion of the invasion. To examine the extent of submucosal invasion, invasion of submucosal layer was divided into three parts: (1) invasion involving the upper 1/3 was regarded as sm1; (2) invasion involving the middle layer as sm2; (3) invasion involving the deepest layer as sm3.(19)
Lymphovascular invasion was defined as the presence of carcinoma cells within endothelial-lined channels. Tumor budding was defined as an isolated single carcinoma cell or a cluster composed of fewer than five carcinoma cells at the invasive front of the tumor. After selection of one field (×200) where budding was the most intensive, a bud count was performed. A field with five or more buds was regarded as positive.(20) Peritumoral inflammation is defined as lymphoid cell infiltration, with or without lymphoid follicle formation immediately adjacent to the tumor, affecting the deepest portion of the carcinoma. Conspicuous or inconspicuous peritumoral inflammation was determined according to the presence of distinctive connective tissue cap at the margin of the tumor in which lymphocytes and other inflammatory cells were scattered.(21)
Sections were dewaxed, rehydrated and washed with PBS. For immunohistochemical stain, sections were heated in a microwave oven at 600 W for 2×5 minutes in 0.01 M citrate buffer at pH 6.0. Sections were immersed in 3% H2O2 to quench endogenous peroxidase activity and non-specific binding was blocked with 5% normal goat serum (0.1% BSA in PBS). Immunohistochemical stain was performed by the avidin-biotin peroxidase complex method with aminoethylcarbazole as a chromogen using the Vectastain ABC elite kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's instructions. Sections were counterstained with Mayer's hematoxylin solution. Primary antibodies for E-cadherin (1:200, mouse monoclonal antibody, Transduction Laboratories, Lexington, KY, USA), β-catenin (1:50, mouse monoclonal antibody, Transduction Laboratories, Lexington, KY, USA), p53 protein (1:50, mouse monoclonal antibody DO-7: DAKO, Carpinteria, CA, USA), Ki-67 (1:150, monoclonal antibody MIB-1: Immunotech SA, Marseille, France), and Smad4 (1:50, goat polyclonal antibody C-20: Santa-Cruz, CA, USA) were used (Fig. 1).
The expression of E-cadherin, β-catenin and Smad4 in tumor cells were compared with their corresponding expression in normal epithelial cells within the same sample. Tumor cells which were immunostained as strongly as the adjacent normal epithelial cells were defined as positive. Cases with over 90% of membranous expression of E-cadherin,(22) were considered to be preserved, while cases with lesser rate of reactivity were regarded as having a reduced expression. β-catenin overall staining density (OSD) was defined as the number of tumor cells with nuclear, cytoplasmic and membranous staining per 100 cells examined. Cases with over 75% of OSD were considered to be high OSD.(23) Presence of Smad4 nuclear staining was defined as positive, while absence of nuclear staining or cytoplasmic staining only was defined as negative.(24) Positive p53 protein expression were considered in cases with over 10% nuclear positive staining in the tumor cells.(25) Ki-67 labeling index were classified as <40 or ≥40 according to the recommended classification in previous study.(26)
Statistical analyses were performed using the chi-square test to estimate the difference in the relationships between the clinicopathologic factors and lymph node metastasis. The Fisher's exact test was used if any expected frequency is less than two or if more than half the expected frequencies are less than five. The Student's t-test was used for statistical comparison of the age and tumor size. Multivariate logistic regression analyses were performed to identify factors with relative importance for lymph node metastasis. All statistical analyses were performed with SPSS version 12.0 for Windows software (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as P of <0.05.
Of the 54 cases with submucosally invasive colorectal carcinoma, lymph node metastases were identified in 13 cases (Table 1). The mean count of sampled lymph node was 14.9 (range 3~57). The mean depth of tumor invasion (±standard deviation) was 2,453.6±1,562.28 µm for all of cases.
The incidence of lymph node metastasis in relation to the clinicopathologic factors evaluated is shown in Table 2. Sex, tumor size, and tumor growth type were not related to lymph node metastasis. Although the differences of the incidences of lymph node metastasis among the groups were not significant, the incidences were higher in patients below 65 years of age, cases with carcinoma located in the rectum, carcinoma with moderate and poor differentiation, sm3 in depth of invasion and conspicuous peritumoral inflammation. The mean age of the cases without lymph node metastasis tended to be older than that of the lymph node-positive group (66.4±11.21 vs. 61.0±7.74, P>0.05). The younger age (<65) group had a higher tendency of having lymph node metastasis compared to the older age (≥65) group (33.3% vs. 16.7%, P>0.05). The incidence of lymph node metastasis in cases with rectal carcinoma was higher than cases with carcinoma located in the colon (30.0% vs. 16.7%, P>0.05). In cases with moderate and poor differentiation, the incidence of lymph node metastasis was higher than that of the cases with well differentiated carcinoma (29.7% vs. 11.8%, P>0.05). The incidence of lymph node metastasis was higher in cases with invasion depth of sm3 as compared to the cases with invasion depth of sm1 and sm2 (33.3% vs. 20.5%, P>0.05). The lymph node metastasis was more common in cases with positive peritumoral inflammation, but was not statistically significant (30.8% vs. 22.0%, P>0.05).
Lymphovascular invasion (55.6% vs. 17.8%, P=0.028) and tumor budding (47.4% vs. 11.4%, P=0.006) were significant clinicopathological predictive indicators of lymph node metastasis in submucosally invasive colorectal carcinoma (Table 2).
The expression status of E-cadherin, β-catenin and p53 in tumor cells was not related to lymph node status. The correlation with Smad4 protein expression and Ki-67 labeling index were suggestive. The incidence of lymph node metastasis was higher in cases that were negative for Smad4 protein expression (28.6% vs. 15.8%, P>0.05) and in cases with Ki-67 ≥40 (28.1% vs. 18.2%, P>0.05). The mean Ki-67 labeling index was similar in the two groups (49.07±31.29 vs. 52.62±25.21, P>0.05) (Table 3).
This present study showed that lymphovascular invasion and tumor budding were significantly related to lymph node metastasis in submucosally invasive colorectal carcinoma. Lymphovascular invasion has been reported as one of the most important risk factors for lymph node metastasis in some studies(27,28); however, the pathologic identification of lymphatic or vascular invasion is difficult. Diagnoses of lymphatic or vascular invasion vary by pathologist and also by the staining method used. Hence, the incidence of lymphovascular invasion may be underestimated or overestimated. Furthermore, retraction or cauterization artifacts may be misdiagnosed as lymphovascular invasion.
A review of the literature has found that some studies classify lymphatic and vascular invasion as a single factor, and these studies have shown that lymphovascular invasion is significantly related to lymph node metastasis.(9,29) Other studies regard lymphatic and vascular invasion two separate factors.(8,27,28) Wang et al.(8) reported that lymphatic invasion was significantly related to lymph node metastasis, but that vascular invasion was not a risk factor. In another study, multivariate analysis showed that vascular invasion was not a risk factor in submucosally invasive colorectal carcinoma, but univariative analysis showed that lymph node metastasis was.(30)
Tumor budding is considered to be a pathologic characteristic corresponding to the initial phase of tumor invasion. Tumor budding is also a significant risk factor in relation to lymph node metastasis in submucosally invasive colorectal carcinoma.(8,9,13) Choi et al.(30) has reported that venous invasion (P=0.021) and tumor budding (P=0.003) are risk factors for lymph node metastasis in submucosally invasive colorectal carcinoma. But by multivariative analysis, only tumor budding was shown to be an independent predictor of lymph node metastasis (P=0.026). The same correlation was identified in our current study. Additionally, in T1 or T2 colorectal carcinoma, tumor budding in combination with lymphovascular invasion was reported to be a useful marker in predicting lymph node metastasis.(31) In the current study, lymph node metastasis was more common in patients with rectal carcinoma than in patients with colon carcinoma (30.0% vs. 16.7%).
Although the cause is unclear, the rectal location of the tumor has been shown to be one of the strong risk factors for lymph node metastasis.(10) Okabe et al.(29) suggested that the higher incidence of lymph node metastasis in rectal carcinoma is due to their intrinsic biology rather than to their location. Their study showed a higher rate of microsatellite stability, aneuploidy, chrosomal deletion, and p53 mutation in rectal and sigmoid cancers than in proximal cancers. We suggest that the bony pelvis with a narrow space around the rectal carcinoma may be related to the higher incidence of lymph node metastasis.
The depth of tumor invasion and level of differentiation have been shown to be risk factors for lymph node metastasis in submucosally invasive colorectal carcinomas.(8,27-29) However, Sohn et al.(9) reported that there was no significant difference in lymph node metastasis according to the depth of tumor invasion and differentiation. In the current study, we also failed to find a significant relationship between lymph node metastasis and the depth of tumor invasion and differentiation, but this lack of correlation may be due by small number of patients in our study.
Studies to find a relationship between immunohistochemical molecular markers, such as E-cadherin, β-catenin. p53 and Ki-67, and lymph node metastasis in submucosally invasive colorectal carcinoma have shown the relationship to be controversial and inconclusive.(11-13) In the current study, we investigated the relationship of the Smad4 protein and lymph node metastatis in submucosally invasive colorectal carcinoma. The Smad4 protein is an important cellular mediator of TGF-β signals relevant for cell development and control of cell growth. It translocates to the nucleus, resulting in the transcription of multiple TGF-β genes, and leading to the regulation of cell proliferation.(14) Recent studies have reported that the mutation of the Smad4 gene or the loss of Smad4 protein expression correlates with tumor progression and metastasis.(15,16) Tanaka et al.(16) have also reported that the loss of Smad4 protein expression is closely related to lymph node metastasis. The process by which the mutation of the Smad4 gene or the loss of Smad4 protein expression affects lymph node metastasis has yet to be shown. However, this study showed that the loss of Smad4 protein expression is associated with a higher incidence of lymph node metastasis in submucosally invasive colorectal carcinoma (28.6% vs. 15.8%, P>0.05), although the correlation was not significant. However, further study is needed to evaluate the predictive value of the loss of Smad4 protein expression.
Additionally, this study showed a higher incidence of lymph node metastasis in submucosally invasive colorectal carcinoma (24.1%) compared with other studies.(5-9) The cause for the higher incidence of lymph node metastasis is unclear. The distribution of tumor location, tumor size, differentiation, depth of tumor invasion, and tumor budding was similar to that of other studies.(8,9,13,28) These results suggest that the incidence of lymph node metastasis in submucosally invasive colorectal carcinoma is not low. Therefore, careful selection is necessary to avoid a curative surgery with lymph node dissection.
In conclusion, lymphovascular invasion and tumor budding were shown to be important predictive factors of lymph node metastasis in submucosally invasive colorectal carcinoma in this study. The correlation between lymph node metastasis and Smad4 mutation and expression was suggestive of the involvement of mutated Smad4 in lymph node metastasis; however, further data are required to evaluate the significance of this correlation.
Figures and Tables
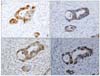 | Fig. 1Immunohistochemical findings. (A) E-cadherin. Membranous and nuclear staining for E-cadherin in tumor cells (×200). (B) β-catenin. Membranous staining for β-catenin in tumor cells (×200). (C) Smad4. Nuclear staining for Samd4 in tumor cells (×200). (D) p53. Nuclear staining for p53in tumor cells (×200). |
Table 2
Correlation between clinicopathologic factors and lymph node metastasis of submucosally invasive colorectal carcinoma

References
1. Kashida H, Kudo SE. Early colorectal cancer: concept, diagnosis, and management. Int J Clin Oncol. 2006. 11:1–8.
2. Hurlstone DP, Sanders DS, Cross SS, Adam I, Shorthouse AJ, Brown S, et al. Colonoscopic resection of lateral spreading tumors: a prospective analysis of endoscopic mucosal resection. Gut. 2004. 53:1334–1339.
3. Williams BM, Saunders BP, Talbot IC. Endoscopic management of polypoid early colon cancer. World J Surg. 2000. 24:1047–1051.
4. Fujimori T, Kawamata H, Kashida H. Precancerous lesion of the colorectum. J Gastroenterol. 2001. 36:587–594.
5. Cranley JP, Petras RE, Carey WD, Paradis K, Sivak MV. When is endoscopic polypectomy adequate therapy for colonic polyps containing invasive carcinoma? Gastroenterology. 1986. 91:419–427.
6. Kyzer S, Begin LR, Gordon PH, Mitmaker B. The care of patients with colorectal polyps that contain invasive adenocarcinoma: endoscopic polypectomy or colectomy? Cancer. 1992. 70:2044–2050.
7. Kikuchi R, Takano M, Takagi K, Fugimoto N, Nozak R, Fujiiyosh T. Management of early invasive colorectal cancer: risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995. 38:1286–1295.
8. Wang HS, Liang WY, Lin TC, Chen WS, Jiang JK, Yang SH, et al. Curative resection of T1 colorectal carcinoma: risk of lymph node metastasis and long-term prognosis. Dis Colon Rectum. 2005. 48:1182–1192.
9. Sohn DK, Chang HJ, Park JW, Choi DH, Han KS, Hong CW, et al. Histopathological risk factors for lymph node metastasis in submucosal invasive colorectal carcinoma of pedunculated or semipedunculated type. J Clin Pathol. 2007. 60:912–915.
10. Nascimbeni R, Burgart LJ, Nivatvongs S, Larson DR. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002. 45:200–206.
11. Hori H, Fujimori T, Fujii S, Ichikawa K, Ohkura Y, Tomita S, et al. Evaluation of tumor cell dissociation as a predictive marker of lymph node metastasis in submucosal invasive colorectal carcinoma. Dis Colon Rectum. 2005. 48:938–945.
12. Makino M, Yamane N, Taniguchi T, Honboh T, Kurayoshi K, Kaibara N. p53 as an indicator of lymph node metastasis in invasive early colorectal cancer. Anticancer Res. 2000. 20:2055–2059.
13. Kaneko I, Tanaka S, Oka S, Yoshida S, Hiyama T, Arihiro K, et al. Immunohistochemical molecular markers as predictors of curability of endoscopically resected submucosal colorectal cancer. World J Gastroenterol. 2007. 13:3829–3835.
14. Heldin CH, Miyazono K, Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997. 390:465–471.
15. Losi L, Bouzourene H, Benhattar J. Loss of Smad4 expression predicts liver metastasis in human colorectal cancer. Oncol Rep. 2007. 17:1095–1099.
16. Tanaka T, Watanabe T, Kazama Y, Tanaka J, Kanazawa T, Kazama S, et al. Loss of Smad4 protein expression and 18qLOH as molecular markers indicating lymph node metastasis in colorectal cancer-a study matched for tumor depth and pathology. J Surg Oncol. 2008. 97:69–73.
17. Shimoda T, Ikegami M, Fujisaki J, Matsui T, Aizawa S, Ishikawa E. Early colorectal carcinoma with special reference to its development de novo. Cancer. 1989. 64:1138–1146.
18. Hamilton SR, Aaltonen LA. WHO Classification of Tumors; Pathology and Genetic of Tumors of the Digestive System. 2000. Lyon: IARC press.
19. Kudo S, Kashida H, Nakajima T, Tamura S, Nakajo K. Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg. 1997. 21:694–701.
20. Ueno H, Mochizuki H, Hashiguchi U, Shimazaki H, Aida S, Hase K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004. 127:385–394.
21. Jass JR, Love SB, Northover JM. A new prognostic classification of colorectal cancer. Lancet. 1987. 1:1303–1306.
22. Kwak JM, Min BW, Lee JH, Choi JS, Lee SI, Park SS, et al. The prognostic significance of E-cadherin and liver intestine-cadherin expression in colorectal cancer. Dis Colon Rectum. 2007. 50:1873–1880.
23. Wanitsuwan W, Kanngurn S, Boonpipattanapong T, Sangthong R, Sangkhathat S. Overall expression of beta-catenin outperforms its nuclear accumulation in predicting outcomes of colorectal cancers. World J Gastroenterol. 2008. 14:6052–6059.
24. Xu WQ, Jiang XC, Zheng L, Yu YY, Tang JM. Expression of TGF-β1, TβRII and Smad4 in colorectal carcinoma. Exp Mol Pathol. 2007. 82:284–291.
25. Giatromanolaki A, Stathopoulos GP, Tsiobanou E, Papadimitriou C, Georgoulias V, Gatter KC, et al. Combined role of tumor angiogenesis, bcl-2, and p53 expression in the prognosis of patients with colorectal carcinoma. Cancer. 1999. 86:1421–1430.
26. Allegra CJ, Paik S, Colangelo LH, Parr AL, Kirsch I, Kim G, et al. Prognostic value of thymidylate synthase, K1-67, and p53 in patients with Dukes\'B and C colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project collaborative study. J Clin Oncol. 2003. 21:241–250.
27. Egashira Y, Yoshida T, Hirata I, Hamamoto N, Akutagawa H, Takeshita A, et al. Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol. 2004. 17:503–511.
28. Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004. 39:534–543.
29. Okabe S, Shia F, Nash G, Wong WD, Guillem FG, Weiser MR, et al. Lymph node metastasis in T1 adenocarcinoma of the colon and rectum. J Gastrointest Surg. 2004. 8:1032–1040.
30. Choi DH, Sohn DK, Chang HJ, Lim SB, Choi HS, Jeong SY. Indication for subsequent surgery after endoscopic resection of submucosally invasive colorectal carcinoma: a prospective cohort study. Dis Colon Rectum. 2009. 52:438–445.
31. Okuyama T, Oya M, Ishikawa H. Budding as a risk factor for lymph node metastasis in pT1 or pT2 well differentiated colorectal adenocarcinoma. Dis Colon Rectum. 2002. 45:628–634.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download