Abstract
Purpose
Diabetes mellitus refers to one of several risk factors for cardiovascular diseases, renal failure and so on. Medical treatments of T2DM cannot suggest a perfect cure. But gastric bypass resulting in the exclusion of the duodenum and proximal jejunum has been shown to improve or resolve T2DM. The goal of this study is to evaluate the effect of duodenojejunal bypass for T2DM patients below BMI 25 kg/m2 in early postoperative period.
Methods
Duodenojejunal bypass was performed on 25 patients at Inha University Hospital from July 2009 to April 2010. We compared 75 g OGTT, insulin, C peptide to those 7 days postoperative. The definitions for improvement are serum glucose level below 200 mg/dl of 75 g OGTT at 120 min or below 200 mg/dl at every other time in spite of over 200 mg/dl at 120 min.
Results
A total of 25 patients (15 men and 10 women) were included. Median value BMI was 23.17 kg/m2 and the mean duration of T2DM was 8.3 years. There was a significant decrease of postoperative 75 g OGTT levels from 176, 268, 345, 373, 371 mg/dl to 125, 170, 200, 225 and 241 mg/dl, respectively (P<0.001). Only patients' age was an independent factor resolution of T2DM based on this study.
Conclusion
Duodenojejunal bypass could be one viable treatment modality for improving or resolving of T2DM although these are early results. This study has preliminary meanings only and the results of longer follow-up and a larger number of patients are necessary, by which we should be able to determine the effect and indications for surgical treatment of T2DM.
Figures and Tables
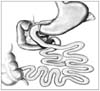 | Fig. 1Duodenojejunal bypass. After transecting jejunum 80 cm from the Treitz ligament, end-to-end anastomosis of distal jejunum and duodenum was made. About 80 cm from this anastomosis, end-to-side jejunostomy was made. |
References
1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998. 21:1414–1431.
2. Joseph AJ, Friedman EA. Diabetic nephropathy in the elderly. Clin Geriatr Med. 2009. 25:373–389.
3. Eliasson B, Eeg-Olofsson K, Cederholm J. Antihyperglycaemic treatment of type 2 diabetes: results from a national diabetes register. Diabetes Metab. 2007. 33:269–276.
4. Pories WJ, Macdonald KG, Flickinger EG. Is type II diabetes mellitus (NIDDM) a surgical disease. Ann Surg. 1992. 215:633–642.
5. Pories WJ, Macdonald KG, Long SB. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995. 222:339–352.
6. Lee WJ, Huang MT, Wang W. Effects of obesity surgery on the metabolic syndrome. Arch Surg. 2004. 139:1088–1092.
7. Madan AK, Orth W, Ternovits CA. Metabolic syndrome: yet another comorbidity gastric bypass helps cure. Surg Obes Relat Dis. 2006. 2:48–51.
8. Mottin C, Padoin AV, Schroer C. Behavior of type 2 diabetes mellitus in morbid obese patients submitted to gastric bypass. Obes Surg. 2008. 18:179–181.
9. Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004. 239:1–11.
10. Rubino F, Forgione A, Cummings DE. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006. 244:741–749.
11. Nathan D. Initial management of glycemia in type 2 diabetes mellitus. N Engl J Med. 2002. 347:1342–1349.
12. Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006. 116:1802–1812.
13. Cohen R, Pinheiro JS, Correa JL. Laparoscopic Roux-en-Y gastric bypass for BMI<35 kg/m2: a tailored approach. Surg Obes Relat Dis. 2006. 2:401–404.
14. Patriti A, Facchiano E, Sanna A. The enteroinsular axis and the recovery from type 2 diabetes after bariatric surgery. Obes Surg. 2004. 14:840–848.
15. Gutniak M, Orskov C, Holst JJ. Antidiabetogenic effect of glucagon-like peptide-1 (7-36) amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992. 326:1316–1322.
16. Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology. 2002. 122:531–544.
17. Gautier JF, Fetita S, Sobngwi E. Biological actions of the incretins GIP and GLP-1 and therapeutic perspectives in patients with type 2 diabetes. Diabetes Metab. 2005. 31:233–242.
18. Ferrannini E, Balkau B. Insulin: in search of a syndrome. Diabet Med. 2002. 19:724–729.
19. Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: Joint Statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005. 28:2289–2303.
20. Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Harnad G. Effect of laparoscopic Roux-en-Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003. 238:467–484.
21. Dixon JB, Pories WJ, O'Brien PE, Schauer P, Zimmet P. Surgery as an effective early intervention for diabestity: why the reluctance? Diabetes Care. 2005. 28:472–474.
22. Nelson KM, Boyko EJ, Koepsell T. All-cause mortality risk among a national sample of individuals with diabetes. Diabetes Care. 2010. 33:2360–2364.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download