Abstract
Purpose
Although progenitor cells may contribute to intimal hyperplasia (IH) after arterial injury, positive contribution of IH is variable with type of injury or cells. This study was designed to examine whether differentiated muscle derived stem cells (MDSC) attenuate IH in rat.
Methods
MDSCs were retrieved using preplate techniques from rat calf muscle and MDSCs (preplate 6th culture fraction, pp6) were exposed to VEGF (50 ng/ml) for endothelial differentiation prior to injection. Male rats were divided into two groups (cell treated vs. control) and underwent carotid balloon injury with 2-Fr catheter. The virus containing Green fluorescent protein (GFP) gene was transfected into cells for monitoring. Cells (5×106) were indwelled into carotid artery for 30 minutes after injury and then blood flow was restored. Arteries were harvested at various intervals (1, 2 and 4 weeks) after injury. The intima to media thickness ratio (IMTR) was calculated with morphometric analysis.
Results
Endothelial surface markers such as VE-CADHERIN were strongly expressed on differentiated MDSCs. At 4 weeks after injury, IH was predominantly observed in control group compared to cell treated group. The intensity of GFP was strongly observed at 1 week and declined at 4 weeks in carotid artery wall at MDSC group. CD31(+) endothelial cells were observed at MDSC group compared to control. The mean IMTR in cell treated groups were significantly lower than control at 2 weeks (P=0.005) and 4 weeks (P≤0.001).
Figures and Tables
Fig. 1
Muscle-derived stem cells (pp6) using preplate technique from rat calf muscle show positive staining with anti-Sca-1 antibody (A), anti-CD34 (B), indeterminate staining with anti-desmin (C) and negative staining with anti-CD45 (D) with immunohistochemical staining (×400).
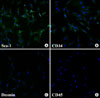
Fig. 2
MDSCs (pp6) are exposed to VEGF for endothelial differentiation. VE-cadherin is up-regulated in dose dependent manner. Values are the mean±SD of three separated experiments.

Fig. 3
Endothelial denudation and intimal hyperplasia are observed in difference following rat carotid balloon injury; Intimal denudation is confirmed in immediately harvested tissue in H&E staining (×100)(A), and in anti-CD31 imunohistochemical staining (×400)(B). Endothelial cell coverage rate in intima is less than 10% in control (C) and 50% in cell-treated group at 1week (D), 30~40% in control (E) and more than 80% in cell-treated group (F) at 2 weeks, 50~70% in control (G) and 90~100% in cell-treated group (H) at 4 weeks after carotid balloon injury. Arrows indicate CD31(+) endothelial cells.
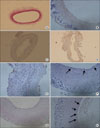
Fig. 4
Contribution of MDSC to intimal hyperplasia. MDSCs infected with lenti-hCMV-GFP were delivered into carotid artery lumen for 30 minutes after carotid balloon injury. MDSCs with GFP (green fluorescent protein) are strongly expressed at 1 week (B) and reduced at 4 weeks in carotid artery (D) compared to controls (A, B). Arrows indicate GFP(+) endothelial cells. MDSCs with CD31 are weakly expressed at 1 week (F) and strongly expressed at 4 weeks in the intima of carotid artery (H) compared to controls (E, G). All sections were viewed at ×100.
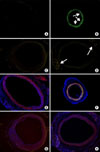
Fig. 5
The intima to media thickness ratio (IMTR) is calculated with morphometric analysis. The mean IMTR in cell treated groups are significantly lower than control at 2 weeks (P=0.005) and 4 weeks (P≤0.001). Black bar indicates control and white bar indicates cell treated group. Values are the mean±SD of five separated experiments.

Fig. 6
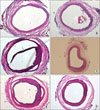
Intimal hyperplasia is inhibited in cell-treated group compared to control group; Intimal hyperplasia is minimally observed in both cell-treated (A) and control group (B) at 1 week. Suppression of intimal hyperplasia is more prominent in cell-treated group (D) than control group (C) at 2 weeks and 4 weeks (E, F). All sections were viewed at ×100.
References
1. Clowes AW, Clowes MM, Fingerle J, Reidy MA. Regulation of smooth muscle cell growth in injured artery. J Cardiovasc Pharmacol. 1989. 14:Suppl 6. S12–S15.
2. Tsai S, Butler J, Rafii S, Liu B, Kent KC. The role of progenitor cells in the development of intimal hyperplasia. J Vasc Surg. 2009. 49:502–510.
3. Fingerle J, Au YP, Clowes AW, Reidy MA. Intimal lesion formation in rat carotid arteries after endothelial denudation in absence of medial injury. Arteriosclerosis. 1990. 10:1082–1087.
4. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997. 275:964–967.
5. Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002. 8:403–409.
6. Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003. 93:783–790.
7. Wang CH, Cherng WJ, Yang NI, Kuo LT, Hsu CM, Yeh HI, et al. Late-outgrowth endothelial cells attenuate intimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arterioscler Thromb Vasc Biol. 2008. 28:54–60.
8. Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002. 105:3017–3024.
9. Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, et al. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004. 109:1769–1775.
10. Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003. 108:2710–2715.
11. Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002. 157:851–864.
12. Huard J. Regenerative medicine based on muscle stem cells. J Musculoskelet Neuronal Interact. 2008. 8:337.
13. Okada M, Payne TR, Zheng B, Oshima H, Momoi N, Tobita K, et al. Myogenic endothelial cells purified from human skeletal muscle improve cardiac function after transplantation into infarcted myocardium. J Am Coll Cardiol. 2008. 52:1869–1880.
14. Huss R, Lange C, Weissinger EM, Kolb HJ, Thalmeier K. Evidence of peripheral blood-derived, plastic-adherent CD34 (-/low) hematopoietic stem cell clones with mesenchymal stem cell characteristics. Stem Cells. 2000. 18:252–260.
15. Tomanek RJ, Schatteman GC. Angiogenesis: new insights and therapeutic potential. Anat Rec. 2000. 261:126–135.
16. Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004. 363:751–756.
17. Na BG, Jang JH. Clinical study for inhibition of intimal hyperplasia: past & present. J Korean Soc Vasc Surg. 2008. 24:155–162.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download