Abstract
Purpose
Muscular artery differs from elastic artery in physical properties and constituents of the arterial wall. To investigate the difference between muscular and elastic arteries, we measured the pulse wave velocities (PWVs) in lower extremity muscular arteries (femoral ankle PWV, faPWV) and abdominal elastic arteries (brachial femoral PWV, bfPWV), and searched for the relationships between the PWVs of muscular, elastic arteries and the risk factors of arteriosclerosis.
Methods
184 normal volunteers were enrolled in the study. Among them, the ratios of male/female, smoker/non-smoker, and hypertension/normal were 81/103, 66/118, and 63/121, respectively. Using volume plethysmography, faPWV and bfPWV were measured. The risk factors of arteriosclerosis in this study were age, gender, smoking, hypertension, body mass index, low density lipoprotein, high density lipoprotein, triglyceride, hemoglobin A1C, and white blood cell.
Results
The PWVs of lower extremity muscular arteries (faPWVs) were significantly faster than those of abdominal elastic arteries (bfPWVs) (right, P<0.001; left, P<0.001) Multiple regression analysis revealed that the independent risk factors of the PWV were age (right, P<0.001; left, P<0.001) and gender (right, P=0.008; left, p=0.014) in abdominal elastic arteries. However, in lower extremity muscular arteries, hypertension (right, P<0.001; left, P<0.001) as well as age (right, P<0.001; left, P<0.001) and gender (right, P=0.009; left, P=0.001) were other significant independent risk factors.
Conclusion
The PWVs of lower extremity muscular arteries were significantly faster than those of abdominal elastic arteries. The significance of hypertension in faPWV suggests that hypertension is an important risk factor in inducing arterial stiffness, especially in lower extremity muscular arteries.
Figures and Tables
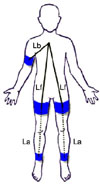 | Fig. 1Measurement of brachial femoral pulse wave velocity (bfPWV) and femoral ankle pulse wave velocity (faPWV). Lb is the distance between the upper portion of sternum and the sensor of right upper arm cuff. Lf is the distance between the upper portion of sternum and the sensors of right and left upper thigh cuffs. La is the distance between the sensors of right and left upper thigh cuffs and the sensors of right and left ankle cuffs. T0 is the moment of the first appearance of the pulse wave from heart at the sensor of right upper arm cuff. Femoral transit time (ΔTf) is the time spent for the pulse wave to pass the distance equivalent to the difference of Lf and Lb. Ankle transit time (ΔTa) is the time spent for the pulse wave to pass the distance of La. Formulae are bfPWV=[(Lf-Lb)/(ΔTf)]×1,000 (cm/sec) and faPWV=[La/(ΔTa-ΔTf)]×1,000 (cm/sec). |
Table 3
Correlation between arteriosclerotic risk factors and pulse wave velocities in 184 participants

*faPWV = femoral ankle pulse wave velocity; †bfPWV = brachial femoral pulse wave velocity; ‡C = pearson correlation coefficient; §P-value = Sig. (2-tailed); ∥Gender (male=1, female=2); ¶Smoking (yes=1, no=2); **Hypertension (yes=1, no=2); ††BMI = body mass index (kg/m2); ‡‡LDL = low density lipoprotein; §§HDL = high density lipoprotein; ∥∥TG = triglyceride; ¶¶HbA1C = hemoglobin A1C (%); ***WBC = number of white blood cell.
References
1. Mircoli L, Mangoni AA, Giannattasio C, Mancia G, Ferrari AU. Heart rate-dependent stiffening of large arteries in intact and sympathectomized rats. Hypertension. 1999. 34:598–602.
2. van der Loop FT, Gabbiani G, Kohnen G, Ramaekers FC, van Eys GJ. Differentiation of smooth muscle cells in human blood vessels as defined by smoothelin, a novel marker for the contractile phenotype. Arterioscler Thromb Vasc Biol. 1997. 17:665–671.
3. Hallett JW, Mills JL, Earnshaw J, Reekers JA, Rooke T. Comprehensive Vascular and Endovascular Surgery. 2009. 2nd ed. Philadelphia: Elsevier.
4. Carmo M, Colombo L, Bruno A, Corsi FR, Roncoroni L, Cuttin MS, et al. Alteration of elastin, collagen and their cross-links in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2002. 23:543–549.
5. Armour BS, Campbell VA, Crews JE, Malarcher A, Maurice E, Richard RA. State-level prevalence of cigarette smoking and treatment advice, by disability status, United States, 2004. Prev Chronic Dis. 2007. 4:A86.
6. Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, et al. Influences of age and gender on results of noninvasive brachial-ankle pulse wave velocity measurement--a survey of 12517 subjects. Atherosclerosis. 2003. 166:303–309.
7. Lee YJ, Lee JW, Kim JK, Lee JH, Kim JH, Kwon KY, et al. Elevated white blood cell count is associated with arterial stiffness. Nutr Metab Cardiovasc Dis. 2009. 19:3–7.
8. Wakabayashi I, Masuda H. Lipoprotein (a) as a determinant of arterial stiffness in elderly patients with type 2 diabetes mellitus. Clin Chim Acta. 2006. 373:127–131.
9. Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension. 2003. 41:183–187.
10. O'Rourke MF, Mancia G. Arterial stiffness. J Hypertens. 1999. 17:1–4.
11. Tieche C, Alkema PK, Liu SQ. Vascular elastic laminae: anti-inflammatory properties and potential applications to arterial reconstruction. Front Biosci. 2004. 9:2205–2217.
12. Eiskjaer H, Christensen T, Pedersen EB. Abnormal structure and increased stiffness of the femoral arterial wall in young patients with sustained essential hypertension. J Intern Med. 1989. 226:235–239.
13. Liu SQ, Moore MM, Yap C. Prevention of mechanical stretch-induced endothelial and smooth muscle cell injury in experimental vein grafts. J Biomech Eng. 2000. 122:31–38.
14. O'Callaghan CJ, Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-beta(1). Hypertension. 2000. 36:319–324.
15. Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation. 1999. 100:1387–1393.
16. Gariepy J, Massonneau M, Levenson J, Heudes D, Simon A. Evidence for in vivo carotid and femoral wall thickening in human hypertension. Groupe de Prevention Cardio-vasculaire en Medecine du Travail. Hypertension. 1993. 22:111–118.
17. Agabiti-Rosei E, Porteri E, Rizzoni D. Arterial stiffness, hypertension, and rational use of nebivolol. Vasc Health Risk Manag. 2009. 5:353–360.
18. Jatoi NA, Mahmud A, Bennett K, Feely J. Assessment of arterial stiffness in hypertension: comparison of oscillometric (Arteriograph), piezoelectronic (Complior) and tonometric (SphygmoCor) techniques. J Hypertens. 2009. 27:2186–2191.
19. Safar ME, Blacher J, Protogerou A, Achimastos A. Arterial stiffness and central hemodynamics in treated hypertensive subjects according to brachial blood pressure classification. J Hypertens. 2008. 26:130–137.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download