Abstract
Purpose
Immunosuppressive regimen based on reduced-dose Tacrolimus (TAC) is widely accepted in the field of renal transplantation. However, optimal targetsfor TAC whole blood trough concentrations during the early period after kidney transplantation remain uncertain.
Methods
A total of 184 consecutive adult renal transplant recipients with triple immunosuppression (TAC/Mycophenolate/corticosteroid) were included in this study. According to the trough level of TAC at day 7 after transplantation, patients were classified as low TAC concentration (LT, <10 ng/ml, n=85), intermediate TAC concentration (IT, 10~15 ng/ml, n=75), and high TAC concentration (HT, >15 ng/ml, n=24) groups. Rate of acute rejection, graft function and side effects of TAC within 1 yr after transplantation were evaluated.
Results
There was no difference in trough concentrations of TAC at 2 weeks, 1 month, 3 months, 6 months and 12 months after transplantation among the three groups. Significantly higher incidence of acute rejection within 2 weeks after transplantation was observed in LT group compared with IT and HT groups (17.4%, 5.6% and 4.8%, respectively, P=0.037). HT patients showed significantly better estimated glomerular filtration rates until 6 months after transplantation than IT and LT patients (75.5±24.8 vs. 63.8±12.8 and 64.3±15.2 ml/min at 6 months, P=0.03). There was no significant difference in TAC toxicity in terms of post-transplant diabetes and renal toxicity.
Figures and Tables
Fig. 1
Changes in tacrolimus trough level. (A) Tacrolimus trough levels were gradually tapered to 6~8 ng/ml at 1 year after transplantation. (B) Patients were classified according to tacrolimus trough level at 7 days after transplantation. LT = low tacrolimus group (FK<10 ng/ml, n=85); IT = intermediate tacrolimus group (FK 10~15 ng/ml, n=75); HT = high tacrolimus group (FK>15 ng/ml, n=24). *P<0.001.

Fig. 2
Incidences of acute rejection. (A) Cumulative probability of acute rejection and (B) frequencies (%) of acute rejection within 2 weeks after transplantation. *P<0.05.

Fig. 3
Changes in eGFR. (A) HT group showed significantly improved graft function until 6 months after transplantation compared to LT and IT patients and (B) until 12 months after transplantation in analysis of LDKT recipients. *P<0.05; †P<0.01.
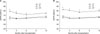
Fig. 4
Tacrolimus toxicity within 1 year after transplantation according to tacrolimus trough levels at 7 days after transplantation. (A) The frequency (%) of tacrolimus renal toxicity identified and (B) Post-transplant diabetes mellitus developed. There were no significant differences among study groups.

Table 2
Univariate and multivariate analysis findings for risk factors of early acute rejection within 2 weeks after kidney transplantation

*The other variables that did not meet criteria (P<0.10) for inclusion into the multivariate analysis were recipient body mass index (P=0.792), retransplantation (P=0.785) and duration of dialysis (P=0.302). †OR = odds ratio; ‡CI = confidence interval; §DDKT = deceased donor kidney transplantation; ∥HLA = human leukocyte antigen; ¶HT = high tacrolimus.
References
1. Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, Barr ML, et al. Immunosuppression: evolution in practice and trends, 1994-2004. Am J Transplant. 2006. 6:1111–1131.
2. Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ. 2005. 331:810.
3. Opelz G, Döhler B. Influence of immunosuppressive regimens on graft survival and secondary outcomes after kidney transplantation. Transplantation. 2009. 87:795–802.
4. Samaniego M, Becker BN, Djamali A. Drug insight: maintenance immunosuppression in kidney transplant recipients. Nat Clin Pract Nephrol. 2006. 2:688–699.
5. Ihara H, Shinkuma D, Ichikawa Y, Nojima M, Nagano S, Ikoma F. Intra- and interindividual variation in the pharmacokinetics of tacrolimus (FK506) in kidney transplant recipients--importance of trough level as a practical indicator. Int J Urol. 1995. 2:151–155.
6. Jusko WJ. Analysis of tacrolimus (FK 506) in relation to therapeutic drug monitoring. Ther Drug Monit. 1995. 17:596–601.
7. Staatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol Dial Transplant. 2001. 16:1905–1909.
8. Borobia AM, Romero I, Jimenez C, Gil F, Ramirez E, De Gracia R, et al. Trough tacrolimus concentrations in the first week after kidney transplantation are related to acute rejection. Ther Drug Monit. 2009. 31:436–442.
9. Kaplan B, Srinivas TR, Meier-Kriesche HU. Factors associated with long-term renal allograft survival. Ther Drug Monit. 2002. 24:36–39.
10. Pascual M, Theruvath T, Kawai T, Tolkoff-Rubin N, Cosimi AB. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002. 346:580–590.
11. Min SI, Yun IJ, Kang JM, Park YJ, Min SK, Ahn C, et al. Moderate-to-severe early-onset hyperuricaemia: a prognostic marker of long-term kidney transplant outcome. Nephrol Dial Transplant. 2009. 24:2584–2590.
12. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999. 55:713–723.
13. Randhawa PS, Shapiro R, Jordan ML, Starzl TE, Demetris AJ. The histopathological changes associated with allograft rejection and drug toxicity in renal transplant recipients maintained on FK506. Clinical significance and comparison with cyclosporine. Am J Surg Pathol. 1993. 17:60–68.
14. Gaspari F, Ferrari S, Stucchi N, Centemeri E, Carrara F, Pellegrino M, et al. Performance of different prediction equations for estimating renal function in kidney transplantation. Am J Transplant. 2004. 4:1826–1835.
15. Lindholm A, Ohlman S, Albrechtsen D, Tufveson G, Persson H, Persson NH. The impact of acute rejection episodes on long-term graft function and outcome in 1347 primary renal transplants treated by 3 cyclosporine regimens. Transplantation. 1993. 56:307–315.
16. Chang SH, Russ GR, Chadban SJ, Campbell SB, McDonald SP. Trends in kidney transplantation in Australia and New Zealand, 1993-2004. Transplantation. 2007. 84:611–618.
17. Ekberg H, Tedesco-Silva H, Demirbas A, Vitko S, Nashan B, Gürkan A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007. 357:2562–2575.
18. Undre NA, van Hooff J, Christiaans M, Vanrenterghem Y, Donck J, Heeman U, et al. Low systemic exposure to tacrolimus correlates with acute rejection. Transplant Proc. 1999. 31:296–298.
19. Kuypers DR, Claes K, Evenepoel P, Maes B, Vanrenterghem Y. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long-term pharmacokinetics in de novo renal allograft recipients. Clin Pharmacol Ther. 2004. 75:434–447.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download