Abstract
Purpose
Methods
Results
Figures and Tables
Fig. 1
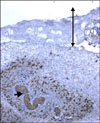
Fig. 2
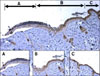
Fig. 3
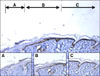
Table 1

Control represents the animals with burn wound dressed with duoderm only. AC group represents the animals with burn wound dressed with aqua cell and duoderm. SF group represent the animals with burn wound dressed with Silk fibroin film and duoderm. *Duncan's multiple range test: Means with the same letter are not significantly different. Means with a different letter are significantly different (P<0.05). Example = A, B are significantly different (P<0.05). Cellularity and Amount of granulation tissue represent the percentage in total area of wound.
Table 2

Control represents the animals with burn wound dressed with duoderm only. AC group represents the animals with burn wound dressed with aqua cell and duoderm. SF group represents the animals with burn wound dressed with Silk fibroin film and duoderm. *Duncan's multiple range test: Means with the same letter are not significantly different. Means with a different letter are significantly different (P<0.05). Example = A, B are significantly different (P<0.05).
Table 3

Control represent the animals with burn wound dressed with duoderm only. AC group represent the animals with burn wound dressed with aqua cell and duoderm. SF group represent the animals with burn wound dressed with Silk fibroin film and duoderm. *Duncan's multiple range test: Means with the same letter are not significantly different. Means with a different letter are significantly different (P<0.05). Example = A, B are significantly different (P<0.05).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download