Abstract
Extraskeletal Ewing' sarcoma (EES) is a rare soft tissue tumor morphologically indistinguishable from the osseous Ewing's sarcoma (ES). We report a case of recurrent EES of the breast that, to the best of our knowledge, has rarely been reported. It was initially confused for a cyst on ultrasound. In addition, MRI and breast-specific gamma imaging (BSGI) in our case have complementary value over conventional imaging.
Ewing's sarcoma (ES) and primitive neuroectodermal tumor (PNET) are the same entity showing varying degrees of neuroectodermal differentiation and they are categorized into a group known as the ES family tumor. Extraskeletal Ewing's sarcoma (EES) arises in soft tissue rather than in relationship to bone. EES/PNET of the breast has been reported in few reports.(1-6) We report a case of recurrent EES of the breast that to best of our knowledge, has rarely been reported. It was initially confused with cyst on ultrasound. In addition, MRI and breast-specific gamma imaging (BSGI) in our case have complementary value over conventional imaging.
A 35-year-old woman was referred to our institute for evaluation of a painless, growing mass in her left breast that she had for 1 month. She underwent lumpectomy of EES in left breast 2 years ago. She complained of rapidly growing, palpable mass at that time and her sonography showed cyst like lesion. Excision specimen included enough safe margins around the tumor. She didn't receive postoperative adjuvant chemotherapy.
Bilateral mammography was performed in our hospital and showed dense breast without evidence of microcalcifications. We performed sonography (ATL HDI 5000; Philips-Advanced Technology Laboratories Ultrasound, Bothell, WA, USA) equipped with 10-mHZ-linear array transducer. Sonography showed several, well-circumscribed, oval anechoic mass with posterior enhancement at the 11~12 o'clock position, 2 cm far from the nipple (Fig. 1A). We initially suspected cysts on the basis of sonographic finding. Because of rapid growing, we biopsied using Mammotome (Johnson & Johnson, New Brunswick, NJ, USA) under sonographic guidance. The pathologic diagnosis was EES.
After biopsy using Mammotome device, she underwent MRI using 1.5-T imaging system (GE Medical Systems, Milwaukee, WI, USA). MRI showed remained, smooth marginated, strong enhancing mass on postcontrast images (Fig. 1B) which showed hyperintense on T2-weighted images (WI) (Fig. 1C) and choline peak on MR spectroscopy (Fig. 1D). BSGI (Dilon 6800 Gamma Camera; Dilon Technologies, Newport News, VA, USA) showed hot uptake in upper inner quadrant (Fig. 1E). Positron emission tomography (PET) and bone scintigraphy showed no uptake.
The patient underwent modified radical mastectomy. Pathology revealed about 2×2-cm, EES and no invasion into skeletal muscle (Fig. 1F). By immunohistochemical staining, tumor cells were positive for CD99, NSE, vimentin, CD56, c-KIT but negative for CD34. She had no evidence of neoplasm in any other part of body. She underwent postoperative chemotherapy consisting vincristine, adriamycin, cyclophosphamide with concurrent radiotherapy. Findings from whole body imaging evaluation were negative for evidence of metastatic disease for 1 year.
EES/PNET is a rare tumor of unknown mesenchymal origin, which predominantly occurs in adolescents and young adults between 10 and 30 years with an equal sex distribution. The principal sites of EES/PNET arises the chest wall, lower extremities, and paravertebral region. EES/PNET generally presents as a rapidly growing, deeply located mass.(4)
High-resolution CT and MRI are useful in the diagnosis and surgical planning, although the features are not specific for EES/PNET. Despite its tendency for local invasion, EES/PNET usually has a pseudocapsule that gives a well-circumscribed appearance. On MRI, these tumors are of high signal intensity on T2-WI, generally of low to intermediated signal intensity on T1-WI and exhibit heterogeneous contrast enhancement.
Simple radiographs of EES/PNET reveal only a nonspecific soft tissue mass. Small areas of amorphous calcifications are not observed in untreated tumor but may develop during chemotherapy. On ultrasound, theses tumors are mostly well-circumscribed, hypoechoic or partially anechoic mass, although a mixed echo pattern may also be recognized.(7)
EES and PNET include a characteristic t(11;22) chromosomal translocation and expression of antigen CD99. Because of the cytogenic and immunohistochemical similarities, EES and PENT are thought to be the same tumor type. For the diagnosis of PNET, some require histologic evidence of neuroectodermal differentiation and others require immunohistochemical evidence of neural markers with or without rosettes. Schmidt et al.(8) suggested that a tumor was designated a PNET if it had rosettes on light microscopy or co-expressed two or more neural markers (NES, S100, chromogranin, synaptophysin, etc) by immunohistochemisty. Using these criteria, our case can be defined as EES rather than PENT, in that tumor showed no rosette formation and were only positive for NES.
In few reports, sonographic finding of breast ES are variable; a superficial, circumscribed hypoechoic mass with posterior enhancement and an apparent tract extending to the skin that was misdiagnoses as an epidermal inclusion cyst, heterogeneous mass with necrotic area that was suspected as cancer.(1-3)
BSGI, known as molecular breast imaging, is breast scintigraphy using a small-filed-of-view γ-camera and 99mTc-sestamibi. BSGI is a cost-effect, highly sensitive and specific technique to detect breast cancer, but breast radiologist should read BSGI imaging in correlation with the mammograms, sonogram, and other imaging studies.
The utility of FDG-PET imaging has not been well established in the diagnosis and staging of soft tissue sarcoma. Our patient was falsely negative on the PET scan. The reason is uncertain but it may be related to differences in the biological behaviors and metabolic profiles.
For the diagnosis of EES/PNET in the breast, the radiologic modality is not specific. If probably benign looking mass grows rapidly without pain, we should consider circumscribed carcinomas such as medullary, mucinous and papillary carcinoma and recommend core biopsy. Medullary carcinomas and mucinous carcinomas of the pure type may mimic a cyst. However, on closer inspection, the margins of theses carcinomas may be irregular, microlobulated.
EES usually follows aggressive course. Because of its rarity, there is a paucity of data on the optical management. Improvement in disease-free-survival was obtained after treatment with chemotherapy, particularly alkylating agents and anthracyclins.
Recurrence of tumors within a short time (less than 2 year) has been explained by some authors as reflecting altered cell kinetics. It can also be speculated that the first tumor was completely eradicated but that other cells in the area, with other growth kinetics predestined to become tumors. This theory can be possibly evoked in patients with a complete resection and adequate craniospinal irradiation.(9)
In summary, we report a rare case in which recurrent primary ES of the breast was initially believed to be probably cyst on the basis of sonographic finding.
Figures and Tables
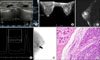 | Fig. 1Primary Ewing's sarcoma of the breast in a 35-year-old woman. (A) Sonography showed a 2 cm, well-circumscribed, oval anechoic mass with posterior enhancement at the 12 o'clock position, 2 cm from the nipple. (B) Maximum intensity projection (MIP) image showed remained, smooth marginated, strong enhancing mass with increased vascularity after IV gadopentetate dimeglumine. (C) The mass showed hyperintense lesion on T2-weighted image. (D) MR spectroscopic image demonstrated elevated choline peak at 3.2 ppm. (E) Breast-specific gamma imaging (BSGI) showed hot uptake in upper inner quadrant (arrow). (F) Immunohistochemically, the lesion is composed of solid sheets of primitive or undifferentiated small round cells (H&E stain, ×200). |
References
1. Maxwell RW, Ghate SV, Bentley RC, Soo MS. Primary primitive neuroectodermal tumor of the breast. J Ultrasound Med. 2006. 25:1331–1333.
2. da Silva BB, Lopes-Costa PV, Pires CG, Borges RS, da Silva RG Jr. Primitive neuroectodermal tumor of the breast. Eur J Obstet Gynecol Reprod Biol. 2008. 137:248–249.
3. Popli MB, Popli V, Bahl P, Solanki Y. Extraskeletal Ewing's sarcoma of the breast. Eur J Radiol Extra. 2009. 70:e65–e67.
4. Ahmad R, Mayol BR, Davis M, Rougraff BT. Extraskeletal Ewing's sarcoma. Cancer. 1999. 85:725–731.
5. Ryu BY, Kim TH, Kim HS, Hwang DJ, Cho JW, Lee HW, et al. Osteogenic sarcoma of the breast. J Korean Surg Soc. 2001. 61:441–444.
6. Ko K, Kim EA, Lee ES, Kwon Y. Primary primitive neuroectodermal tumor of the breast: a case report. Korean J Radiol. 2009. 10:407–410.
7. Enzinger FM, Weiss SW. Enzinger FM, Weiss SW, editors. Extraskeletal Ewing's sarcoma/primitive neuroectodermal tumor family. Soft Tissue Sarcoma. 2001. 4th ed. St. Louis: Mobis;1289–1308.
8. Schmidt D, Herrmann C, Jürgens H, Harms D. Malignant peripheral neuroectodermal tumor and its necessary distinction from Ewing's sarcoma. A report from the Kiel Pediatric Tumor Registry. Cancer. 1991. 68:2251–2259.
9. Lefkowitz IB, Packer RJ, Ryan SG, Shah N, Alavi J, Rorke LB, et al. Late recurrence of primitive neuroectodermal tumor/medulloblastoma. Cancer. 1988. 62:826–830.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download