Abstract
Purpose
The clinical advantages of end-to-end (ETE) anastomosis have not been clear despite its biomechanical advantage over end-to-side (ETS) anastomosis. We compared the histomorphometric features of intimal remodeling after ETE and ETS anastomosis in a rabbit aortic bypass model.
Methods
Thirty-two bypass operations, 16 with ETS and 16 with ETE anastomoses, were performed using aortic allografts of donor rabbits (15 per group) and polytetrafluoroethylene (PTFE) grafts (1 per group). To minimize bias from the immunologic response to aortic allografts or graft size, a long aortic tissue obtained from one donor was divided into 2 pieces and shared between each ETE and ETS bypass. PTFE graft bypasses, which are commonly used in clinical practice, were performed to provide comparison results for an allograft with a different compliance. Vessels were harvested at 1 day (1 per group), 5 days (1 per group), and 4 weeks (14 per group, including the PTFE bypass group) after surgery. Intimal thickening was evaluated with hematoxylin-eosin, van Gieson, immunohistochemical staining and Western blot analysis of TNF-α and proliferative cell nuclear antigen (PCNA) expression.
Results
Mean intimal thickness and volume (0.721±0.047 mm, 5.734±0.387 mm3 vs. 0.883±0.048 mm, 9.068±0.462 mm3) and intima/media volume ratio (0.70±0.05 vs 1.08±0.06) were significantly smaller in ETE (P<0.05). Western blotting showed a marked increase in TNF-α (203.15±5.29 vs. 494.49±6.11) and PCNA concentrations (152.66±7.37 vs. 175.53±4.36) in the ETS group.
Intimal hyperplasia (IH), also referred to as neointimal proliferation, is a major component of vascular remodeling and is responsible for the occlusion following angioplasty, stenting, endarterectomy, and bypass procedures.(1) Induction and progression of IH are thought to involve mechanisms related to wall shear stress (WSS), platelet aggregation/activation, the nature of the conduit, and neointimal proliferation after metabolic or stress-induced cellular injury, such as lipid disorders, hypertension, and tobacco use.(2-4)
Aortobifemoral bypass remains an important treatment for aortoiliac occlusive disease, despite the evolution in endovascular techniques.(5) In an aortobifemoral or aortobiiliac bypass, either end-to-end (ETE) or end-to-side (ETS) might be chosen for proximal anastomosis. In vitro studies have shown that, ETE anastomoses appear to offer a biomechanical advantage over ETS anastomoses with regard to WSS within the anastomotic region.(6,7) Experimentally, however, slow recirculation flow and the occurrence of anastomotic intimal thickening have also observed after ETE anastomosis.(8) Likewise, stenosis or occlusion at the proximal suture site of ETE anastomosis of aortobifemoral bypass is not infrequently observed as well as ETS anastomosis. We therefore compared the histomorphometric features of intimal remodeling after ETE and ETS anastomosis, the distribution of neointima between the bypass grafts and artery, and cytokine expression, in a rabbit model.
In accordance with national laws on animal experiments, and with the permission of our university Ethics Committee, we utilized male New Zealand White rabbits, weighing 2.0~2.5 kg and aged 8~10 weeks (Orient, Sungnam, Korea). A total of 32 aorto-aortic bypass operations of 16 with ETS and 16 with ETE anastomosis were performed. Thirty operations were done using aortic grafts of 15 donor rabbits and 2 operations polytetrafluoroethylene (PTFE: Gore-Tex; W.L. Gore and Associates) grafts. To minimize bias from the immunologic response to aortic allografts or different graft size, a long aortic tissue obtained from one donor was divided into 2 pieces and shared between each of ETE and ETS bypass. PTFE graft bypasses, which are commonly used in clinical practice, were performed to provide comparison results for an allograft with a different compliance. Vessel wall changes were assessed at 1 day (n=1 per group), 5 days (n=1 per group), and 4 weeks (n=14 per group, including 1 per group operated with the PTFE graft).(9) Prior to harvesting of aortic segments, the rabbits were monitored daily, and their behavior, food and water intake, and neurological alterations were recorded.
All animals were anesthetized with a combination of ketamine hydrochloride (10 mg/kg, Ketamine; Yuhan, Seoul, Korea) and xylazine hydrochloride (3 mg/kg, Rompun; Bayer Korea, Suwon, Korea), and maintained unconscious with inhaled isoflurane (Aerane; Baxter, Deerfield, IL, USA).(10) After shaving the abdomen and disinfecting the skin, the animals were prepared and positioned, and a median 6 cm abdominal incision was made. In donor rabbits, the aorta was identified and exposed by sharp dissection, and its branches were ligated with 5-0 black silk. After completing dissection and mobilization of the aortic segment, heparin (120 U/kg) was given intravenously prior to aortic clamping. Each harvested aortic graft was washed with heparinized saline and stored at 4℃ in histidine-tryptophan-ketoglutarate solution. After skin closure with nylon 3-0, donor animals were euthanized in a CO2 chamber. In the ETS anastomosis group, an arteriotomy measuring approximately 3 mm in length was made at the ventral surface of the aorta. The donor aorta or a PTFE graft, measuring approximately 9 mm in length, was anastomosed to the recipient aorta, using 8-0 polypropylene sutures and a continuous suture technique (Fig. 1A).(9) Upon completion of the anastomosis, the distal end of the recipient's aorta was ligated and transected. The distance between the caudal end of the anastomosis and the ligated terminus was 3 mm. The distal end of the transected aorta was anastomosed to the end of the aortic graft. ETE anastomoses with 8-0 polypropylene sutures were performed between the proximal and the distal end using a conti nuous suture technique (Fig. 1B). The length of each graft was approximately 6 mm.
After restoring the blood flow, the abdominal incision was closed with a running 4-0 Maxon skin suture. Recovery was aided by use of ketorolac tromethamine (Tarasyn; Roche Korea, Seoul, Korea) for 3 days.
At 1~28 days after the surgical procedure, the animals were anesthetized as described above, and the skin incisions were reopened. On mobilizing the aorta, the upper abdominal aorta was tied off after assessment of bypass patency, and cannulated distally to prevent shrinkage from fixation. A segment of the aortic graft containing the anastomosis, and a 3~4 mm portion of control vessel on either side were dissected from the surrounding tissues, and fixed for further histological assessment. After skin closure with nylon 3-0, the animals were euthanized in a CO2 chamber. The surgery sites were macroscopically assessed for stenoses and thrombi around the anastomosis, and sites where such features occurred were noted. Segments were stained with hematoxylin-eosin (H-E) or van Gieson stain for morphological evaluation of the anastomoses, identification of inflammatory cells and visualization of hyperplasic changes in the neointima. The neointima-to-media volume ratio was also quantified using imaging software (ImageJ; NIH, Bethesda, MD, USA).(11)
Anti-mouse proliferative cell nuclear antigen (PCNA) (clone PC 10, 1:1,000 dilution) (Sigma Chemical, St. Louis, MO, USA) and anti-mouse tumor necrosis factor-alpha (TNF-α) (dilution, 1:1,000) (Cell Signaling Technology Inc., Danvers, MA, USA) antibodies were used for immunohistochemical analyses. After blocking of endogenous peroxidase activity with 0.5% hydrogen peroxide in methanol for 5 min at room temperature, specimens were preincubated for 30 min with 10% goat serum in phosphate-buffered saline for 30 min, and then incubated at room temperature for 30 min with monoclonal antibodies against PCNA and TNF-α. Each vessel wall was divided into eight equal sections, and all cells within the media and intima were examined. Nonspecific cytoplasmic staining without nuclear involvement was considered negative. The PCNA labeling index was determined by dividing the number of PCNA-positive nuclei by the total number of nuclei in the neointima and media from each section. The intensity of anti-TNF-α staining of the intima and media of the eight randomly chosen sections was measured using imaging software (ImageJ). The medial and neointimal volume, PCNA indices and mean gray scale of TNF-α staining of the two groups were compared.
TNF-α and PCNA expression in three pair of bypass grafts explanted 4 weeks after surgery was analyzed by Western blotting. Each aortic segments bearing proximal anastomosis of 2 cm in length were snap-frozen, ground into powder, and lysed in 1 ml of 10 mM Tris-HCL (pH 7.4) containing 150 m-mol/L NaCl, 1% Triton X-100, and 1 mM phenylmethylsulfonyl-fluoride. The lysates were centrifuged and the supernatants subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by transfer to nitrocellulose membranes, and incubation with anti-mouse TNF-α (dilution, 1:1,000) (Cell Signaling Technology Inc.) and anti-mouse PCNA (clone PC 10, 1:1,000 dilution) (Sigma Chemical) antibodies. Expression was quantitated using imaging software (ImageJ).
Intima and media thickness and volumes were compared using the Mann-Whitney U-test. Statistical analysis was performed using the statistical package SPSS for Windows (Version 12.0, SPSS Inc., Chicago, IL, USA). Statistical significance was defined when a P-value of <0.05 was obtained.
There was no graft occlusion or limb ischemia during 4 weeks of follow-up. Four weeks after bypass, the intimal proliferation were clearly recognized by H-E and van Gieson's staining (Fig. 2). The intima became thicker and had higher cellularity with typical spindle-shaped cells, and cellular proliferation around the suture thread disappeared. Whereas the neointima in ETE anastomoses were concentric, we observed a thick cellular layer, containing abundant extracellular matrix, on the ventral surface and heel of grafts that employed ETS anastomoses (Fig. 3).
Table 1 summarizes the light microscopy results for intimal thickness and volume. The thickness, area, and volume of neointima differed significantly between the two groups. Mean intimal thickness (0.883±0.048 mm vs. 0.721±0.047 mm) and mean neointimal volume (9.068±0.462 mm3 vs. 5.734±0.387 mm3) were significantly greater in the ETS group (P<0.05 for each comparison) (Fig. 2). Mean medial volume was, however, similar in the ETE (8.309±0.378 mm3) and ETS (8.446±0.316 mm3) groups. Using PTFE grafts, the neointimal maximal thickness and volume were 0.12 mm and 1.11 mm3, respectively, in the ETE group, and 0.84 mm and 3.62 mm3, respectively, in the ETS group (Fig. 4). Similarly, the intima/media volume ratio (0.70±0.05 vs. 1.08±0.06) was significantly smaller in ETE than in the ETS group (P<0.05). The circumference (15.853±0.306 mm vs. 24.877±0.817 mm) and area (15.364±0.382 mm2 vs. 24.419±1.157 mm2) of the lumen were significantly larger in the ETS than in the ETE group (P<0.05 for each comparison), but the intimal volumes of distal anastomoses did not differ significantly (7.63±0.042 mm3 vs. 7.98±0.030 mm3, P>0.05).
Immunohistochemical staining for PCNA and TNF-α showed a significant increase in the number of proliferating cells in the neointima of ETS grafts compared with the ETE anastomosis group. The PCNA index in the intima and media was also significantly lower in the ETE than in the ETS anastomosis group (6.01±2.01% vs. 17.22±6.12%, P<0.05) (Fig. 5). The intensity of TNF-α staining, calculated by use of a mean gray scale, was significantly weaker in the ETE group (150.29±6.57 vs. 125.67±3.12, P<0.05) (Fig. 5).
Western blotting of extracts of three entire bypass grafts 4 weeks after surgery showed significantly lower PCNA (152.66±7.37 vs. 175.53±4.36) and TNF-α (203.15±5.29 vs. 494.49±6.11) in the ETE group (Fig. 6).
Late graft failure caused by progressive IH is a persistent complication in arterial reconstruction, with IH accounting for 30~50% of bypass graft failures.(12) Implantation of a vein or prosthetic graft in the arterial circulation induces various changes that may lead to IH, including increased shear stress, loss of endothelial cells, migration and invasion of inflammatory cells, and migration and proliferation of vascular smooth muscle cells (VSMC).(13)
Pathologically, stenotic lesions after graft bypass have been shown to consist of VSMC proliferation with consequent luminal narrowing.(14) We assayed expression of PCNA and TNF-α to determine which cells had been stimulated to undergo proliferation. We found that medial VSMC proliferation occurred more when the ETS rather than the ETE anastomotic method was employed.
Activated leukocytes are an important trigger of VSMC proliferation in the development of luminal stenosis and occlusion. One of the cytokines strongly implicated in VSMC proliferation is TNF-α, an important mediator of both systemic and local inflammatory responses to vascular injury, and responsible for VSMC proliferation in a variety of settings including bypass surgery, arterial balloon injury and acute cardiac rejection.(15) In vitro, TNF-α induces adhesion molecule expression, promotes monocyte cytokine release, and stimulates VSMC proliferation.(16) In vivo, TNF-α has been causally linked to IH in vein graft and low-shear-stress models.(15,17) We observed significant differences in PCNA and TNF-α expression, by both immunohistochemical staining and Western blotting, between ETS and ETE anastomoses.
Since the aortic allografts in our model had good compliance, the effects of mismatched compliance and consequent angulation between the graft and host arteries were likely minimal. Therefore, we compared the pathologic findings of aortic allografts to those of the PTFE model, and the results were consistent. An abrupt increase in luminal size caused by ETS anastomosis, as shown by measuring the circumference and area of the lumen, may have resulted in the observed between-group difference in the degree and morphology of IH; the lesions were eccentric, and thickest on the ventral portion, in ETS anastomoses, but concentric in ETE anastomoses. This finding suggests that angulation of the anastomosis may have caused flow disturbance and subsequent IH. These data also suggest that ETS may have increased proliferative activity around the anastomosis and such anastomoses may be more prone to stenosis than are ETE anastomoses, and that both flow pattern and direction are important in late-phase IH formation. However, proximal anastomoses did not affect the degree of IH at distal anastomoses, and the anastomotic method seemed to have only a localized impact on the development of IH. At the same time, these changes were confined to sites near the anastomosis, suggesting that they were not related to an immunologic reaction to the allograft.
Although we found that ETE was superior to ETS anastomosis in reducing the rate of IH, clinical results have been mixed. Whereas some studies reported higher patency rates for ETE anastomosis after aortobifemoral or aortobiiliac bypass,(5,18) others found no difference.(19,20) ETE anastomoses have the theoretical advantage of less turbulence at the anastomosis; furthermore, the graft does not protrude anteriorly against the duodenum, and the graft limbs are less likely to kink. ETE anastomosis was considered preferable, in that angulation at the origin of the limbs of the prosthesis was found in ETS grafts.(21) In addition, the distal aorta, as the most common site for aneurysms and a potential source of emboli from ulcerated plaques, can be excluded.(22) The advantages of ETS anastomosis include technical simplicity and preservation of the inferior mesenteric artery; moreover, if graft occlusion occurs, there may be sufficient flow through the iliac arteries to prevent limb loss.(18) Furthermore, there may be an increased risk of impotence following ETE anastomosis.(22) Therefore, when choosing of proximal anastomotic method in arterial occlusive disease, the clinical parameters, including patient age, side branches near the anastomosis, and luminal size and appearance of the artery at operation should be considered with the surgical method associated with longer graft patency, at the same time.
We have developed an aortic bypass model in rabbits by interposing aortic allografts into the abdominal aorta using different anastomotic methods. The aorta used for both allografts and bypass sites had very thin intima, and changes in endothelial cells soon after surgery were difficult to detect by light microscopy. However, abundant intimal proliferation was readily visualized within 4 weeks. In addition, the aorta of a rabbit aged 8~10 weeks is approximately 2~2.2 mm in diameter and can be readily handled using a surgical loupe. Moreover, the anastomoses could be performed with running sutures.(9) Furthermore, ETE anastomosis was possible without raising concerns regarding distal organ ischemia, including colon and limb ischemia or paraplegia if the bypass was performed on the distal aorta near the bifurcation.
In conclusion, at 4 weeks, neointima developed mostly around the ventral surface in the ETS anastomosis group and circumferentially in the ETE anastomosis group. ETE anastomosis yielded significantly better results than did ETS anastomosis with respect to decreased IH, and may therefore improve long-term graft patency.
Figures and Tables
Fig. 1
Operative field. (A) End-to-side anastomosis. (B) End-to-end anastomosis. The white arrow indicates the ligated end of the host artery.
Fig. 2
Analysis of anastomoses 4 weeks after implantation. H-E (A) and van Gieson staining (B), showing thicker neointima in the end-to-side anastomosis group. Arrowheads indicate the internal elastic lamina (Original magnification: ×400).
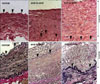
Fig. 3
End-to-side anastomosis 4 weeks after implantation. Side walls (A) and ventral surface (B, C) of the grafts. Thicker cellular layer containing abundant extracellular matrix were observed on the ventral surfaces. Arrowheads indicate the internal elastic lamina (H-E, Original magnification: A, C, ×400; B, ×100).

Fig. 4
Van Gieson staining of proximal anastomoses of the polytetrafluoroethylene (PTFE) bypass group. Thicker neointima in end-to-side (B) than endto-end anastomosis graft (A) was observed. Arrowheads indicate theneointima (Original magnification: left two panels ×100; right two panels ×400).
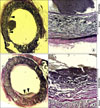
Fig. 5
Immunohistochemical staining for proliferating cell nuclear antigen (PCNA) and tumor necrosis factor-alpha (TNF-α) in proximal anastomoses of rabbit aortic grafts 4 weeks after implantation. PCNA-positive cells (black arrow) were more abundant in the end-to-side (B) than end-to-end (A) anastomosis group, as was diffuse immunostaining for TNF-α throughout the neointima/media. Arrowheads indicate internal elastic lamina (PCNA = proliferating cell nuclear antigen; TNF-α = tumor necrosis factor-alpha) (Original magnification: left two panels, ×200, and right four panels, ×400).
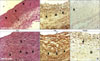
Fig. 6
Western blotting of proteins of three aortic grafts containing proximal anastomosis 4 weeks after implantation. Quantitative analysis showed that expression of proliferating cell nuclear antigen (PCNA) (A) and tumor necrosis factor-alpha (TNF-α) (B) was higher in the end-to-side (ETS) group than in the end-to-side (ETS) group. Results are expressed as means±SEMs (PCNA = proliferating cell nuclear antigen; TNF-α = tumor necrosis factor-alpha).

References
1. Sottiurai VS. Can intimal hyperplasia and distal anastomotic intimal hyperplasia be controlled and prevented? Ann Vasc Surg. 2007. 21:289–291.
2. Loth F, Jones SA, Zarins CK, Giddens DP, Nassar RF, Glagov S, et al. Relative contribution of wall shear stress and injury in experimental intimal thickening at PTFE end-to-side arterial anastomoses. J Biomech Eng. 2002. 124:44–51.
3. Schwartz SM, Campbell GR, Campbell JH. Replication of smooth muscle cells in vascular disease. Circ Res. 1986. 58:427–444.
4. Trubel W, Schima H, Czerny M, Perktold K, Schimek MG, Polterauer P. Experimental comparison of four methods of end-to-side anastomosis with expanded polytetrafluoroethylene. Br J Surg. 2004. 91:159–167.
5. Brewster DC, Darling RC. Optimal methods of aortoiliac reconstruction. Surgery. 1978. 84:739–748.
6. Sottiurai VS, Sue SL, Feinberg EL 2nd, Bringaze WL, Tran AT, Batson RC. Distal anastomotic intimal hyperplasia: biogenesis and etiology. Eur J Vasc Surg. 1988. 2:245–256.
7. Kim YH, Chandran KB, Bower TJ, Corson JD. Flow dynamics across end-to-end vascular bypass graft anastomoses. Ann Biomed Eng. 1993. 21:311–320.
8. Ishibashi H, Sunamura M, Karino T. Flow patterns and preferred sites of intimal thickening in end-to-end anastomosed vessels. Surgery. 1995. 117:409–420.
9. Schachner T, Laufer G, Bonatti J. In vivo (animal) models of vein graft disease. Eur J Cardiothorac Surg. 2006. 30:451–463.
10. Frevert CW, Matute-Bello G, Martin TR. Rabbit models of pneumonia, peritoneal sepsis, and lung injury. Methods Mol Biol. 2000. 138:319–330.
11. Dello SA, van Dam RM, Slangen JJ, van de Poll MC, Bemelmans MH, Greve JW, et al. Liver volumetry plug and play: do it yourself with ImageJ. World J Surg. 2007. 31:2215–2221.
12. Imparato AM, Bracco A, Kim GE, Zeff R. Intimal and neointimal fibrous proliferation causing failure of arterial reconstructions. Surgery. 1972. 72:1007–1017.
13. Ishida M, Komori K, Yonemitsu Y, Taguchi K, Onohara T, Sugimachi K. Immunohistochemical phenotypic alterations of rabbit autologous vein grafts implanted under arterial circulation with or without poor distal runoff-implications of vein graft remodeling. Atherosclerosis. 2001. 154:345–354.
14. Swedberg SH, Brown BG, Sigley R, Wight TN, Gordon D, Nicholls SC. Intimal fibromuscular hyperplasia at the venous anastomosis of PTFE grafts in hemodialysis patients. Clinical, immunocytochemical, light and electron microscopic assessment. Circulation. 1989. 80:1726–1736.
15. Faries PL, Marin ML, Veith FJ, Ramirez JA, Suggs WD, Parsons RE, et al. Immunolocalization and temporal distribution of cytokine expression during the development of vein graft intimal hyperplasia in an experimental model. J Vasc Surg. 1996. 24:463–471.
16. Pober JS, Bevilacqua MP, Mendrick DL, Lapierre LA, Fiers W, Gimbrone MA Jr. Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986. 136:1680–1687.
17. Rectenwald JE, Moldawer LL, Huber TS, Seeger JM, Ozaki CK. Direct evidence for cytokine involvement in neointimal hyperplasia. Circulation. 2000. 102:1697–1702.
18. Madiba TE, Mars M, Robbs JV. Choosing the proximal anastomosis in aortobifemoral bypass. Br J Surg. 1997. 84:1416–1418.
19. Mellière D, Labastie J, Becquemin JP, Kassab M, Paris E. Proximal anastomosis in aortobifemoral bypass: end-to-end or end-to-side? J Cardiovasc Surg (Torino). 1990. 31:77–80.
20. Ameli FM, Stein M, Aro L, Provan JL, Gray R, Grosman H. End-to-end versus end-to-side proximal anastomosis in aortobifemoral bypass surgery: does it matter? Can J Surg. 1991. 34:243–246.
21. Gaylis H. Aorto-iliac by-pass grafting end-to-end or end-to-side anastomosis. S Afr J Surg. 1973. 11:45–49.
22. Pierce GE, Turrentine M, Stringfield S, Iliopoulos J, Hardin CA, Hermreck AS, et al. Evaluation of end-to-side v end-to-end proximal anastomosis in aortobifemoral bypass. Arch Surg. 1982. 117:1580–1588.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download