Abstract
Purpose
The genetic polymorphism and intracellular activity of methylenetetrahydrofolate reductase (MTHFR) and thymidylate synthase (TS) is clinically associated with carcinogenesis and biological therapeutic effect in gastrointestinal malignancies. We aimed to elucidate the susceptibility of gastric cancer according to MTHFR and TS gene polymorphism.
Methods
This study was designed as a hospital-based case-control study in a single institute. The gastric cancer group (n=300) for the study was diagnosed at first time as tubular adenocarcinoma, and the control group (n=100) was diagnosed as no malignancy in the endoscopic biopsy. The genetic polymorphism of TS and MTHFR were confirmed by PCR.
Results
The MTHFR mutant type had a more than 2-fold increased risk of developing gastric cancer (RR: 2.341). But, only heterozygote type (677CT) revealed significantly higher susceptibility compared to wild type (RR: 2.581). In TS gene genotype, the mutant genotype rate (2R/3R and 3R/3R) was significantly higher in gastric cancer group compared to control group (P=0.008), and the mutant type had a more than 3-fold increased risk of developing gastric cancer (RR: 3.222). In combined MTHFR and TS, 677CT+2R/3R and 677CT+3R/3R there was more than a 3-fold increased risk rate of developing gastric cancer compared with other combinations (RR, 3.474 in 677CT with 2R/3R; RR, 3.895 in 677CT with 3R/3R).
Gastric cancer is a major cause of death worldwide, although there are differences of its prevalence between Asian countries and Western countries. The molecular events for the carcinogenesis of gastric cancer have been studied in detail, yet there are increasing concerns about the genetic polymorphism of gastric cancer. Several attempts are currently being made to search for screening tools, prognostic factors, and markers that can predict the therapeutic response during chemotherapy.(1,2) However, any meaningful markers associated with the diagnosis or therapeutic response for gastric cancer has not yet been found.
Several studies have reported that low levels of dietary folic acid are associated with an increased risk for gastrointestinal cancer.(3,4) DNA methylation, which has an important role in carcinogenesis, can be modified by limiting the supply of methyl groups in the diet.(5,6) An important biological function of folate is to provide the methyl groups which are required for intracellular methylation reactions and de novo deoxynucleoside triphosphate synthesis. Therefore, folate deficiency is thought to be carcinogenic through the disruption of DNA methylation/synthesis and impairment of DNA repair.(7)
Methylenetetrahydrofolate reductase (MTHFR) is an enzyme that catalyzes the intracellular reduction of 5,10-methylenetetrahydrofolate (5,10-MTHF) to 5-methylenetetrahydrofolate (5-MTHF) as a key protein in the DNA methylation pathway. A common polymorphism in the MTHFR gene is a cytosine (C) at codon 677 is replaced by a thymine (T), and there are three genotypes; 677CC (wild type), 677CT (heterozygote mutant) and 677TT (homozygote mutant). The homozygote mutant has recently been reported to be associated with increased plasma homocysteine, but only in the condition with low plasma folate levels. Another genetic mutation of the MTHFR gene is the substitution of the 1,098th base pair. The biological concentration or activity of MTHFR can be decreased in the circumferences of folic acid deficiency or genetic mutation of the MTHFR gene. But if the MTHFR gene mutation will occur despite an adequate amount of folic acid intake, then the activity of MTHFR will also be decreased.(8,9)
Thymidylate synthase (TS) is a key enzyme in the nucleotide synthesis that catalyzes the methylation of deoxyuridine monophosphate (dUMP) by 5,10-MTHF acting as a cofactor. This function is very important not only as a source of thymidylate in the cell, but also for the DNA replication and repair system.(10) This enzyme has drawn interest as a primary target for cancer chemotherapeutic agents such as 5-fluorouracil (5-FU), 5-fluoro-2-prime-deoxyuridine and some folate analogs. The majority of the polymorphisms of TS are originated from the phenomena of double-tandem repeat (2R) or triple-tandem repeat (3R) of the TS enhancer region (TSER), and there are three genotypes: 1) the wild type 2R/2R, 2) the heterozygote mutant 2R/3R and 3) the homozygote mutant 3R/3R.(11) A higher rate of the repeated number has revealed a greater rate of expressing TS mRNA. The polymorphism of the TS gene affects disturbing the level of intracellular folic acid, which results in an imbalance of the ratio of dUMP/dTMP, and this can be associated with the susceptibility to a specific cancer by causing an error in DNA replication.(12)
It has been reported that the polymorphism of TS or MTHFR is related with the risk of esophageal, endometrial and breast cancer.(13-15) Moreover, the intracellular activity of TS and MTHFR is clinically associated with the biological therapeutic effect of 5-FU-based chemoregimens for most gastrointestinal malignancies because the main mechanism of 5-FU is inhibition of TS by fluorodeoxyuridine monophosphate, which forms an inactive complex with TS and 5,10-MTHF. The activity of both enzymes is under genetic control and several gene polymorphisms are known to influence their tissue expression.(16)
In this study, we aimed to determine the susceptibility of Koreans to gastric cancer according to MTHFR and TS gene polymorphism.
Briefly, this study was designed as a hospital-based case-control study in a single institute. The patients of the gastric cancer group (n=300) for the study were diagnosed for the first time as having tubular adenocarcinoma from January, 2003 to June, 2007 at St. Mary's Hospital, The Catholic University of Korea. The patients of the control group (n=100) were diagnosed as not having malignancy on their endoscopic biopsies. The cases with synchronous malignancies or inaccurate medical records were excluded from the analysis. All the patients provided their written informed consent for participation in the study.
The venous blood was collected from the subjects in both groups. The DNA was extracted from the lymphocytes using a DNA extraction kit (QIAmp® DNA Mini Kit, QIAGEN Inc., Hilden, Germany). The extracted DNA was standardized to 100 ng and 1µl was used with 1 pmol of the primer 10 mM of dNTP, 0.5 units of Taq DNA polymerase and 2µl of 10× PCR buffer solution (100 mM Tris-HCl, 500 mM KCl, 15 mM MgCl2); a total mixture of 20µl was used for the PCR. The T-1 Thermoblock PCR machine (Biometra, Germany) was used for PCR.
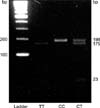
The sense primer was 5'-TGA AGG AGA AGG TGT CTG CGG GA-3' and the antisense primer was 5'-AGG ACG GTG CGG TGA GAG TC-3'. In order to amplify the 198 bp products, after 5 minutes of early denaturation at 95℃ followed by 15 seconds of denaturation at 95℃, 60 seconds of annealing at 56℃ and 60 seconds of extension reaction at 50℃ were repeated for 40 cycles. The restriction enzyme Hinf I can recognize the CT sequence of MTHFR on the amplified fragments. After being treated with Hinf I, the fragments were checked by running them on a 2.5% agarose gel. The MTHFR codon 677 mutational variant was analyzed by PCR-RFLP and three types were found. The 677CC (wild type) was not degraded by Hinf I and only the 198 bp fragment was shown. For the 677CT (heterozygote type) genotype, 198 bp, 175 bp and 23 bp fragments were seen, and 175 bp and 23 bp fragments were observed for the 677TT (homozygote type) genotype (Fig. 1).
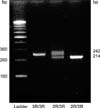
The sense primer was 5'-GTG GCT CCT GCG TTT CCC CC-3', and the antisense primer was 5'-GGC TCC GAG CCG GCC ACA GGC ATG GCG CGG-3'. After the initial denaturation at 94℃ for five minutes, the DNA was denatured at 94℃ for forty seconds, then an annealing process was done at 62℃ for 1 minute and 35 cycles for 40 seconds was done for the extension reaction; finally, the sample was reacted at 72℃ for 5 minutes. The electrophoresis of the amplified DNA fragments was performed in 4% agarose gel for 30 minutes. The homozygotic double repeat 2R/2R (wild type) formed a single band at 214 bp and the heterozygotic 2R/3R (heterozygote type) formed double bands at 214 bp and 242 bp, and finally the homozygotic triple repeat 3R/3R (homozygote type) formed a single band at 242 bp (Fig. 2).
The analysis was performed according to each of the gene genotypes, and only the combined status was inputted into the multivariate analysis. The results of the continuous variables are expressed as means±standard deviation (SD). Univariate statistical analysis was performed using Chi-square or Fisher's exact test for the categorical variables, and the binary logistic regression method was used for the multivariate analysis. Statistical analyses were performed using SPSS software (Ver. 13.0) and a P-value<0.05 was considered to indicate a statistically significant difference with a 95% confidence interval (95% CI).
In the gastric cancer group, the ages of the patients ranged from 14 to 91 years old (mean age: 59.3±12.4 years) and the male/female ratio was 181/119. In the control group, the patients' ages ranged from 12 to 74 year old (mean age: 45.8±16.0 years) and the male/female ratio was 35/65 in the control group. The cancer group was significantly older than control in age, and male gender was significantly dominant in cancer group (P<0.05, data not shown). The distribution of gastric cancer patients according to the sixth edition UICC/TNM staging system were as follows: IA, 119 (29.8%); IB, 60 (15.0%); II, 37 (9.3%); IIIA, 21 (5.3%); IIIB, 18 (4.5%); IV, 45 (11.3%) (data not shown).
The distribution of each gene polymorphism is shown in detail in Table 1. For the MTHFR gene polymorphism, the frequencies for MTHFR 677CC, 677CT and 677TT were 6.0%, 72.7% and 21.3%, respectively, for the gastric cancer group and they were 13.0%, 61.0% and 26.0%, respectively, for the normal control group. The heterozygote genotype (677CT) was revealed to have the highest rate in the gastric cancer group and the control group in a similar fashion (72.7% and 61.0%, respectively). The expressed frequency of the 3 genotypes was revealed to be significant different (P=0.031). The mutant type (667CT and 667 TT) was showed to occur at a significantly higher rate in the gastric cancer group as compared to that of the normal group (P=0.023). For the TS gene polymorphism, the frequencies for TS 2R/2R, 2R/3R and 3R/3R were 3.3%, 30.3% and 66.3%, respectively, for the gastric cancer group and 10.0%, 30.3% and 60.0%, respectively, for the normal control group. The homozygote genotype (3R/3R) was revealed to have the highest rate in the gastric cancer group (66.3%), and this was similar to that in the control group (60.0%). There was a significant difference in the expression rate of the three genotypes of the TS gene between the gastric cancer group and the control group (P=0.028). The mutant genotype (2R/3R and 3R/3R) was present at a significantly higher rate in the gastric cancer group as compared to that of the control group (P=0.008) (Table 2).
The multivariate analysis result for the susceptibility to gastric cancer is shown in Table 3. First, for the MTHFR gene genotypes, the subjects with the mutant type had more than a 2-fold increased risk of gastric cancer (RR: 2.341, 95% CI: 1.103~4.970) as compared with that of the subjects with the MTHFR wild genotype. Yet only the heterozygote type (677CT) was revealed to confer a significantly higher susceptibility to gastric cancer as compared to that of the wild type (RR: 2.581, 95% CI: 1.198~5.562). For the TS gene genotype, the subjects with the mutant type had more than a 3-fold increased risk of gastric cancer (RR: 3.222, 95% CI: 1.300~7.988) as compared with that of the subjects with the wild genotype. Among the mutant types, the homozygote type (3R/3R) showed a higher risk than that of the heterozygote type (RR: 3.317 vs 3.033, respectively).
For the combination of the MTHFR and TS, the 677CT+3R/3R type revealed highest rate in the gastric cancer group (49.3%), similar to that in the control group (38.0%). Interestingly, the wild type had the lowest expression rate in both groups. A combination of 677CT+2R/3R and 677CT+3R/3R had a more than 3-fold increased risk of gastric cancer compared with that of the other combinations (RR: 3.474, 95% CI: 1.004~12.019 in the 677CT with 2R/3R; RR: 3.895, 95% CI: 1.189~12.756 in the 677CT with 3R/3R, respectively) (Table 3).
Genetic polymorphism makes a difference for the susceptibilities of specific malignancies between individuals or ethnic groups. Though genetic polymorphism is not a single major cause for carcinogenesis, it is able to increase the risk as a co-factor in the circumstance of interaction with environmental factors. If a genetically highly sensitive group for a certain cancer could be examined according to their genetic polymorphism, more effective screening and helpful prevention should be possible in the future. Among the diverse genetic polymorphisms, a specific polymorphism associated with metabolic enzymes causes a difference in intracellular enzymatic activity.(17) Moreover, the polymorphism of the gene associated with methylation can result in carcinogenesis because DNA methylation has been implicated in the pathophysiology of many cancers in the aspect of epigenetics.
In this report, we focused on MTHFR and TS gene polymorphism in normal and cancer population prior to evaluating the possibility of using these marker to predict its responsibility for a response to 5-FU in gastric cancer patients in the Korean population. MTHFR and TS gene polymorphism affects DNA synthesis and repair systems, and plays an important role in carcinogenesis by inhibition of DNA methylation.(18)
The biological concentration or activity of MTHFR can be decreased in the circumstance of folic acid deficiency or genetic mutation of the MTHFR gene. One of the important things associated with MTHFR is the gene-nutrient interaction. However, in the presence of low folate intake, both impaired DNA methylation and DNA synthesis/repair may become the primary mechanism of carcinogenesis in those people who have the variant MTHFR genotypes.(13) Therefore, folate deficiency is also thought to be carcinogenic through the disruption of DNA methylation/synthesis and impaired DNA repair.(7) Whereas the wild type of the MTHFR genotype (667CC) had full enzymatic activity, the heterozygote (677CT) and homozygote (677TT) mutant genotypes showed 65% and 30% activity, respectively. It was reported that diminution of MTHFR activity resulted in mismatching of uracil during DNA synthesis, breaking of the DNA's double strand and inhibition of DNA methylation. Consequently, these genetic events could be resulted in epigenetic carcinogenesis.(19) The potential clinical implications resulting from the lower genomic DNA methylation observed in persons, who are presented MTHFR 677TT, are less clear.(20) In our study, the wild type MTHFR group paradoxically was lowest for cancer, and the expression rate of the heterozygote was higher than the expression rate of the homozygote that has less enzymatic activity. However, the heterozygote MTHFR mutant group (677CT) had only relatively higher susceptibility to gastric cancer as compared to that of the normal population and the difference was significant (RR=2.581, P=0.016). The 667TT mutant type did not have a relative risk for gastric cancer as compared to that of the 667CT mutant (P=0.183). For esophageal squamous cell carcinoma (ESCC), an elevated ESCC risk associated with the 677 polymorphism showed an allele-dose relationship in the Chinese population.(13) Several other studies have reported an association of MTHFR and the cancer risk. One study reported a 50% reduction in the colorectal cancer risk with the TT genotype compared to persons with the wild type MTHFR.(21) On the other hand, it was recently reported that the TT genotype becomes a risk factor for colorectal adenoma under the condition of low folate intake.(20) However, we don't have the data for folate intake in this study, and further study is necessary about this point for clarification.
The TS is located on the short arm of the 18th chromosome, and is a folate-dependent enzyme that plays an important role in the biosynthesis of thymidylate, which is an important precursor nucleotide for de novo DNA synthesis. Once MTHF becomes dihydrofolate, it induces the transfer of a methyl group, and converts dUMP to dTMP in DNA synthesis. It was reported that the expression of the TS gene with 2R was 3.6-fold lower than that of 3R, and the TS mRNA level in the 2R/2R genotype was 3.6-fold lower than that of the 3R/3R genotype in the cancer tissue.(11,22) In our study, the 2R/3R and 3R/3R genotypes were found more frequently in the gastric cancer group with significance, and the heterozygote and homozygote TS mutant group had only relatively higher susceptibility to gastric cancer as compared with that of the normal population with significance (RR=3.033 in 2R/3R, RR=3.317 in 3R/3R). However, it was reported that there was few limitations for explaining the cancer susceptibility because a comparison of the 2R/2R and 3R/3R genotypes might have provided stronger evidence of differential translation with an unknown mechanism, and the 2R/2R genotype is quite rare in Asians.(12) It was also reported that the homozygous (3R/3R) mutant was found to have elevated levels of intra-tumoral TS mRNA, but the enzyme activity was not matched with the genotype.(23) In the tissue of metastatic colon cancer, the activity of the TS in the 3R/3R was far greater than in the 2R/3R.(24) However, because there are many normal cells in gastric cancer tissue, false positive or negative results could frequently occur when quantitatively analyzing the TS mRNA or proteins. These results also support that TSER genotyping is more useful than assessing mRNA or protein due to its lower error rate. The TS genotype information may be a useful predictor of the efficacy of TS-related chemotherapy. The principle is that greater levels of TS translation could protect cells from the cytotoxic effect of 5-FU. The induction of the TS protein expression through autoregulation after 5-FU exposure was proposed as one mechanism for tumor resistance to 5-FU. Moreover, TS protein induction with no change of the TS mRNA expression shortly after 5-FU exposure has been observed in many cell lines and clinical samples.(25,26)
The TS is a key enzyme in the nucleotide synthesis that catalyzes the methylation of dUMP by 5,10-MTHF as a cofactor, and a correlation of genetic polymorphism between the two genes could be existed. Therefore, we postulate that the combination of both genomic polymorphisms could be increased the susceptibility to gastric cancer, and we evaluated the combined results of each genes. The combination of 677CT+2R/3R and 677CT+3R/3R had a more than 3-fold increased risk of gastric cancer as compared with that of the other combinations. There have been reported no other comparable results up to now.
However, this study has some limitations. There were significant differences in age and gender between the groups because this study was designed as case-control study, and the basic mutation rate in both groups was higher compared to previously reported results. We think that the dissimilarity of mutation rate has been resulted from the difference of diet by regional, but this correlation should be confirmed in the next.
In conclusion, this study shows a significant association between the MTHFR and TS polymorphism and the susceptibility to gastric cancer, and provides a genetic basis for this relationship. Moreover, MTHFR and TS polymorphisms could be used as genetic marker under a specific dietary (folate) environment. In addition, a large scale patient-control cohort study is needed to confirm the biological function that involves such ethnic and geographical aspects.
Figures and Tables
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the help of Jae Im Lee, M.D. for performing laboratory work.
References
1. El-Rifai W, Powell SM. Molecular biology of gastric cancer. Semin Radiat Oncol. 2002. 12:128–140.
2. Gonzalez CA, Sala N, Capella G. Genetic susceptibility and gastric cancer risk. Int J Cancer. 2002. 100:249–260.
3. Gonzalez CA, Riboli E, Badosa J, Batiste E, Cardona T, Pita S, et al. Nutritional factors and gastric cancer in Spain. Am J Epidemiol. 1994. 139:466–473.
4. Kim YI. Methylenetetrahydrofolate reductase polymorphisms, folate, and cancer risk: a paradigm of gene-nutrient interactions in carcinogenesis. Nutr Rev. 2000. 58:205–209.
5. Pogribny IP, Basnakian AG, Miller BJ, Lopatina NG, Poirier LA, James SJ. Breaks in genomic DNA and within the p53 gene are associated with hypomethylation in livers of folate/methyl-deficient rats. Cancer Res. 1995. 55:1894–1901.
6. Kim YI, Pogribny IP, Basnakian AG, Miller JW, Selhub J, James SJ, et al. Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am J Clin Nutr. 1997. 65:46–52.
7. Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr. 2000. 130:129–132.
8. Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998. 64:169–172.
9. Nelen WL, Blom HJ, Thomas CM, Steegers EA, Boers GH, Eskes TK. Methylenetetrahydrofolate reductase polymorphism affects the change in homocysteine and folate concentrations resulting from low dose folic acid supplementation in women with unexplained recurrent miscarriages. J Nutr. 1998. 128:1336–1341.
10. Mandola MV, Stoehlmacher J, Muller-Weeks S, Cesarone G, Yu MC, Lenz HJ, et al. A novel single nucleotide polymorphism within the 5' tandem repeat polymorphism of the thymidylate synthase gene abolishes USF-1 binding and alters transcriptional activity. Cancer Res. 2003. 63:2898–2904.
11. Kawakami K, Salonga D, Park JM, Danenberg KD, Uetake H, Brabender J, et al. Different lengths of a polymorphic repeat sequence in the thymidylate synthase gene affect translational efficiency but not its gene expression. Clin Cancer Res. 2001. 7:4096–4101.
12. Trinh BN, Ong CN, Coetzee GA, Yu MC, Laird PW. Thymidylate synthase: a novel genetic determinant of plasma homocysteine and folate levels. Hum Genet. 2002. 111:299–302.
13. Song C, Xing D, Tan W, Wei Q, Lin D. Methylenetetrahydrofolate reductase polymorphisms increase risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Res. 2001. 61:3272–3275.
14. Esteller M, Garcia A, Martinez-Palones JM, Xercavins J, Reventos J. Germ line polymorphisms in cytochrome-P450 1A1 (C4887 CYP1A1) and methylenetetrahydrofolate reductase (MTHFR) genes and endometrial cancer susceptibility. Carcinogenesis. 1997. 18:2307–2311.
15. Campbell IG, Baxter SW, Eccles DM, Choong DY. Methylenetetrahydrofolate reductase polymorphism and susceptibility to breast cancer. Breast Cancer Res. 2002. 4:R14.
16. Gusella M, Frigo AC, Bolzonella C, Marinelli R, Barile C, Bononi A, et al. Predictors of survival and toxicity in patients on adjuvant therapy with 5-fluorouracil for colorectal cancer. Br J Cancer. 2009. 100:1549–1557.
17. Gonzalez FJ. The role of carcinogen-metabolizing enzyme polymorphisms in cancer susceptibility. Reprod Toxicol. 1997. 11:397–412.
18. Stern LL, Mason JB, Selhub J, Choi SW. Genomic DNA hypomethylation, a characteristic of most cancers, is present in peripheral leukocytes of individuals who are homozygous for the C677T polymorphism in the methylenetetrahydrofolate reductase gene. Cancer Epidemiol Biomarkers Prev. 2000. 9:849–853.
19. Guttormsen AB, Ueland PM, Nesthus I, Nygard O, Schneede J, Vollset SE, et al. The Hordaland Homocysteine Study. Determinants and vitamin responsiveness of intermediate hyperhomocysteinemia (> or =40 micromol/liter). J Clin Invest. 1996. 98:2174–2183.
20. Ulrich CM, Kampman E, Bigler J, Schwartz SM, Chen C, Bostick R, et al. Colorectal adenomas and the C677T MTHFR polymorphism: evidence for gene-environment interaction? Cancer Epidemiol Biomarkers Prev. 1999. 8:659–668.
21. Ma J, Stampfer MJ, Giovannucci E, Artigas C, Hunter DJ, Fuchs C, et al. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res. 1997. 57:1098–1102.
22. Kawakami K, Omura K, Kanehira E, Watanabe Y. Polymorphic tandem repeats in the thymidylate synthase gene is associated with its protein expression in human gastrointestinal cancers. Anticancer Res. 1999. 19:3249–3252.
23. Pullarkat ST, Stoehlmacher J, Ghaderi V, Xiong YP, Ingles SA, Sherrod A, et al. Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J. 2001. 1:65–70.
24. Etienne MC, Chazal M, Laurent-Puig P, Magne N, Rosty C, Formento JL, et al. Prognostic value of tumoral thymidylate synthase and p53 in metastatic colorectal cancer patients receiving fluorouracil-based chemotherapy: phenotypic and genotypic analyses. J Clin Oncol. 2002. 20:2832–2843.
25. Omura K, Kawakami K, Kanehira E, Nagasato A, Kawashima S, Tawaraya K, et al. The number of 5-fluoro-2'-deoxyuridine-5-monophosphate binding sites and reduced folate pool in human colorectal carcinoma tissues: changes after tegafur and uracil treatment. Cancer Res. 1995. 55:3897–3901.
26. Peters GJ, van Triest B, Backus HH, Kuiper CM, van der Wilt CL, Pinedo HM. Molecular downstream events and induction of thymidylate synthase in mutant and wild-type p53 colon cancer cell lines after treatment with 5-fluorouracil and the thymidylate synthase inhibitor raltitrexed. Eur J Cancer. 2000. 36:916–924.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download