Abstract
Purpose
Cyclooxygenase-2 (Cox-2) is an inducible enzyme that converts arachidonic acid to prostaglandins. Aberrant expression of Cox-2 and prostaglandins has been observed in many cancers, including colon and breast cancers, and 40% of human breast cancers show overexpression of Cox-2. The aim of this study was to analyze the role of Cox-2 expression in breast cancers.
Methods
The expression of Cox-2 and HER2 was examined in 56 breast tissue samples including microscopically normal epithelium and invasive ductal carcinomas (IDC) using immunohistochemical (IHC) methods. Frozen breast cancers and adjacent non-cancerous tissue (ANCT) pairs (n=30) were analyzed for Cox-2 and HER2 mRNA expression by RT-PCR. The results were compared with the prognostic parameters of breast cancer including tumor grade, growth pattern, lymph node metastasis, estrogen receptor status and Ki-67 labeling index.
Results
Cytoplasmic Cox-2 expression was detected in 39 of 56 (69.6%) IDC and the Cox-2 expression in IDC was closely associated with HER2 expression (P=0.023). However, the expression of Cox-2 was not associated with other prognostic parameters of breast cancer (P>0.05). The Cox-2 mRNA showed high expression levels in IDC (25/30, 83.3%) as well as ANCT (22/25, 88%).
Conclusion
The association between the expression of Cox-2 and HER2 suggests that Her2/neu gene induces the Cox-2 expression in breast cancer and overexpression of Cox-2 is involved in breast cancer development. Though the cells of ANCT are normal in morphology, their molecular alteration (overexpression of Cox-2) suggests that these cells have transformed already.
Figures and Tables
Fig. 1
Photographs showing the degrees of cytoplasmic protein expression of Cox-2; 0 (A), 1+ (B), 2+ (C) and 3+ (D).
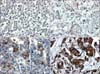
Fig. 2
Photographs showing laser capture microdissection from adjacent non-cancerous tissue (A) and the cells collected in CapsureTM (B). Microdissection from cancer (C) and the cells collected (D).
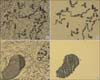
Fig. 3
Photomicrograph showing representative RT-PCR results for Cox-2 and HER2 in normal epithelium (N) and IDC (T).

References
1. Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985. 312:146–151.
2. Cardiff RD, Muller WJ. Transgenic mouse models of mammary tumorigenesis. Cancer Surv. 1993. 16:97–113.
3. Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004. 56:387–437.
4. Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998. 12:1063–1073.
5. Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000. 69:145–182.
6. Araki Y, Okamura S, Hussain SP, Nagashima M, He P, Shiseki M, et al. Regulation of cyclooxygenase-2 expression by the Wnt and ras pathways. Cancer Res. 2003. 63:728–734.
7. Wu T. Cyclooxygenase-2 and prostaglandin signaling in cholangiocarcinoma. Biochim Biophys Acta. 2005. 1755:135–150.
8. Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994. 107:1183–1188.
9. Ristimäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997. 57:1276–1280.
10. Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999. 59:198–204.
11. Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998. 58:3761–3764.
12. Half E, Tang XM, Gwyn K, Sahin A, Wathen K, Sinicrope FA. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002. 62:1676–1681.
13. Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996. 274:2057–2059.
14. Lakhani SR, Chaggar R, Davies S, Jones C, Collins N, Odel C, et al. Genetic alterations in 'normal' luminal and myoepithelial cells of the breast. J Pathol. 1999. 189:496–503.
15. Sozzi G, Miozzo M, Tagliabue E, Calderone C, Lombardi L, Pilotti S, et al. Cytogenetic abnormalities and overexpression of receptors for growth factors in normal bronchial epithelium and tumor samples of lung cancer patients. Cancer Res. 1991. 51:400–404.
16. Limpens J, de Jong D, van Krieken JH, Price CG, Young BD, van Ommen GJ, et al. Bcl-2/JH rearrangements in benign lymphoid tissues with follicular hyperplasia. Oncogene. 1991. 6:2271–2276.
17. Pinkas-Kramarski R, Eilam R, Alroy I, Levkowitz G, Lonai P, Yarden Y. Differential expression of NDF/neuregulin receptors ErbB-3 and ErbB-4 and involvement in inhibition of neuronal differentiation. Oncogene. 1997. 15:2803–2815.
18. DiAugustine RP, Richards RG, Sebastian J. EGF-related peptides and their receptors in mammary gland development. J Mammary Gland Biol Neoplasia. 1997. 2:109–117.
19. Normanno N, Ciardiello F. EGF-related peptides in the pathophysiology of the mammary gland. J Mammary Gland Biol Neoplasia. 1997. 2:143–151.
20. Schroeder W, Biesterfeld S, Zillessen S, Rath W. Epidermal growth factor receptor-immunohistochemical detection and clinical significance for treatment of primary breast cancer. Anticancer Res. 1997. 17:2799–2802.
21. Krane IM, Leder P. NDF/heregulin induces persistence of terminal end buds and adenocarcinomas in the mammary glands of transgenic mice. Oncogene. 1996. 12:1781–1788.
22. Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001. 37:Suppl 4. S9–S15.
23. Vadlamudi R, Mandal M, Adam L, Steinbach G, Mendelsohn J, Kumar R. Regulation of cyclooxygenase-2 pathway by HER2 receptor. Oncogene. 1999. 18:305–314.
24. Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem. 2002. 277:18649–18657.
25. Witton CJ, Hawe SJ, Cooke TG, Bartlett JM. Cyclooxygenase 2 (COX2) expression is associated with poor outcome in ER-negative, but not ER-positive, breast cancer. Histopathology. 2004. 45:47–54.
26. Tan KB, Yong WP, Putti TC. Cyclooxygenase-2 expression: a potential prognostic and predictive marker for high-grade ductal carcinoma in situ of the breast. Histopathology. 2004. 44:24–28.
27. Ranger GS, Jewell A, Thomas V, Mokbel K. Elevated expression of cyclooxygenase-2 in breast cancer and ductal carcinoma in situ has no correlation with established prognostic markers. J Surg Oncol. 2004. 88:100–103.
28. Liu CH, Chang SH, Narko K, Trifan OC, Wu MT, Smith E, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001. 276:18563–18569.
29. Piazza GA, Rahm AL, Krutzsch M, Sperl G, Paranka NS, Gross PH, et al. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995. 55:3110–3116.
30. Sharpe CR, Collet JP, McNutt M, Belzile E, Boivin JF, Hanley JA. Nested case-control study of the effects of non-steroidal anti-inflammatory drugs on breast cancer risk and stage. Br J Cancer. 2000. 83:112–120.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download