Abstract
Purpose
A study was designed to assess the effect of intraperitoneal instillation of ropivacaine in larparoscopic cholecystectomy patients using computerized patient controlled anesthesia (PCA).
Methods
From January 2009 to June 2009, 40 patients with uncomplicated, symptomatic cholecystitis with cholelithiasis who were referred to Chung-Ang University Medical Center for laparoscopic cholecystectomy were included in the study. Patients in group C (control group) received normal saline 100 ml and those in group I (instillation group) received intraperitoneal instillation of 2 mg/kg of ropivacaine diluted in 100 ml saline at the initiation of pneumoperitoneum. Patients were assessed for pain by blinded investigators at 6 time intervals after surgery; 2 hr, 4 hr, 8 hr, 12 hr, 24 hr, and 48 hr. The frequency at which patients pushed the button of the PCA on bolus requirement (FPB) was assessed by a patient-controlled module on the PCA machine.
Results
The mean total fentanyl consumption was lower in group I (367.39±85.88) than in group C (535±100.29) during the 48 hours (P<0.001). Fentanyl velocity and FPB showed significant difference between the groups (P<0.005). Visual analogue scale (VAS) measured pain scores were significantly lower in group I than in group C at 4 hr (P=0.027), 8 hr (P=0.010), 12 hr (P=0.011).
Laparoscopic cholecystectomy (LC) is the treatment of choice in treating gallbladder disease substituting the conventional open method of cholecystectomy (OC). LC has improved surgical outcome in terms of reduced pain, morbidity and duration of convalescence compared to OC.(1-3) However, while reduced postoperative pain is the most worthy achievement from the patient's point of view LC is not a pain-free procedure, which is why many LC patients refrain from early recovery to normal activity and imperils the feasibility of LC at stake.(4-6) Because LC is one of the most common surgical procedures in Korea performed over 20,000 case/year (2007 National Statistical Office, Korea), the socioeconomic influences from prolonged recovery is considerable.
Previous studies advocate the etiology of postoperative pain in patients who underwent LC is multifactorial consisting visceral pain from the operation itself, parietal pain originating from the trauma to diaphragm as well as the peritoneum and the incision pain itself.(3,7-10) The trials of controlling the pain from incision sites were undertaken but the results are controversial and evidence suggest that dominant pain from the procedure lies in peritoneum rather than skin and abdominal wall.(4,11-13) Whereas clinicians have investigated beneficial methods to reduce parietal and visceral pains by additive procedures during operation.(2,5,7,9,10,14)
In this study we designed a prospective double-blind randomized control study to assess the effect of intraperitoneal ropivacaine instillation during operation and analyze precise pain pattern and opioid amount from computerized patient controlling anesthesia (PCA).
Randomized double-blind placebo-controlled study was designed and carried out according to the principles of Declaration of Helsinki, 1989. Written consent was obtained from all participants before inclusion in the trial.
From January 2009 to June 2009, 40 patients with uncomplicated, symptomatic cholecystitis with cholelithiasis who referred to Chung-Ang University Medical Center for LC were included in the study. Patient ages below 18 years or over 65 years, weight under 45 kg or over 100 kg, underlying disease of severe cardiovascular, renal, hepatic diseases, and allergy to local anesthetics were excluded from the population.
Patients in group C (control group) received normal saline 100 ml and those in group I (instillation group) received intraperitoneal instillation of 2 mg/kg ropivacaine diluted in 100 ml saline at the initiation of pneumoperitoneum.
Randomization in two groups (group C, group I) were based on Excel random number generation. The detail of series were unknown to investigators and contained in a set of sealed envelope, each bearing on the outside only the number. In the operation room, just before induction of anesthesia, the appropriate numbered envelope was opened and instillation was prepared according to the card inside indicating patient group C or I. The operator entered the room after instillation preparation and performed procedure without information. The postoperative pain data was collected by blinded investigators.
All patients were referred to operating room without premedication. Non invasive blood pressure, pulse oxymetry, electrocardiogram were monitored all through the anesthetic period. Anesthesia was induced with 4~5 mg/kg thiopental, 0.6 mg/kg rocuronium and 2µg/kg fentanyl. After tracheal intubation, general anesthesia was maintained with 2.0~3.0% v/v sevoflurane of end-tidal concentration. During the surgery, patients received intravenous infusion of lactated Ringer's solution at a rate of 5~7 ml/kg/hr. No additional intravenous opioid was injected during the surgery.
All the surgical procedures were performed by a single surgeon. 11 mm trocar umbilical port was introduced by closed method using Veres needle. Under visual confirmation, epigastric port was created at the right side border of falciform ligament by 11 mm trocar and just 2 finger widths below the inferior costal margin in the midclavicular line and midaxillary line. Intraperitoneal pressure was maintained at 12~15 mmHg during the operation which was routine procedure and the operative table was placed in reverse Trendelenburg position with left-side down tilting position.
In both groups, immediately after the creation of pneumoperitoneum under appropriate laparoscopic visual field, instillation was performed through a catheter via second trocar. This procedure was repeated 10 min prior to initiation of manipulating the gallbladder, the operator sprayed 50 ml of solution on the upper surface of liver dome and on the right sub-diaphragmatic space, and other 50 ml of solution around liver bed of the future cholecystectomized site. After instillation procedure, in order to obtain thorough diffusion 2 minutes of Trendelenburg position was maintained.
Patients were delivered solid meal from the following day of the operation and complaints of intolerable indigestion, nausea, vomiting and other gastrointestinal symptoms were recorded. To control postoperative pain, the computerized intravenous patient controlled analgesia (PCA, Automed 3300™, Ace Medical Co., Seoul, Korea) with fentanyl was used. The mode of PCA was continuous infusion, 0.1µg/kg/hr; bolus 0.1µg/kg; lockout interval, 15 min (total regimen 100 ml).
Whenever pain occurs, the patients were taught to push freely on the button on the PCA which delivers the bolus drug, and when pain exceeding visual analogue scale (VAS) pain score 30 mm persists, fentanyl 50µg was injected via intravenous route till the pain relieved under VAS 30 mm as a rescue analgesic treatment at each outcome measure time.
For each patient, we recorded age, gender, American Society of Anesthesiologists (ASA) class, anesthesia time (from injection of thiopental to extubation), operation time (from incision to skin suture). To measure the pain intensity, VAS (0~100 mm) was used. Patients were assessed for pain by blinded investigators at 6 time intervals after surgery; 2 hr, 4 hr, 8 hr, 12 hr, 24 hr, and 48 hr. The dose of rescue fentanyl and fentanyl delivered by PCA was measured and summated as fentanyl consumption. The frequency to push the button of PCA on bolus requirement (FPB) was assessed by patient controlled module of the PCA machine at the same time intervals.
To calculate the required study size, we took into account VAS pain score in the pilot study. Standard deviation of VAS pain score in pilot study was 10 mm. We accepted a 2-tailed α error of 5% and β error of 10% to detect a difference of the VAS pain score 10. Based on these calculations the required study size per group was 16. Considering compliance rate as 80%, we divided 20 patients per group in this study.
For continuous variables, differences between study groups were compared using unpaired t-test. The descriptive variables were analyzed either by chi-square analysis or Fischer's exact test, as appropriate. P<0.05 was considered statistically significant. Data in the figures are reported as mean values with standard error. Statistical analysis was performed with SPSS version 13.0 (SPSS, Chicago, IL, USA).
Among the 40 patients, all 40 outcomes were eligible for the study without any resignations nor PCA discontinuation for any cause. During the total study no vomiting or intolerable indigestion was observed. Forty patients were randomized in 2 groups in equal size. There were no significant differences (Table 1) in respect of age, sex, body mass index (BMI), ASA class, anesthesia time and also the operation time between the groups. All patients underwent elective LC without major complication such as hemorrhage, bile duct injury, open conversion.
The mean total fentanyl consumption was lower in the group I (367.39±85.88) than in group C (535±100.29) during the 48 hours (P<0.001) (Fig. 1). Comparing each velocity of consumed fentanyl per hour, every interval time of estimation showed significant difference between the groups (P<0.005) (Table 2, Fig. 2).
VAS scores were significantly lower in group I than C at 4 hr (P=0.027), 8 hr (P=0.010), 12 hr (P=0.011). The differences showed no statistical difference in 24 hr (P=0.191), and 48 hr (P=0.255) after the operation (Table 2, Fig. 3).
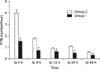
The computerized frequency count of patient controlled bolus injection showed significant difference between the groups throughout the time of estimation on 4 hr (P<0.001), 8 hr (P<0.001), 12 hr (P=0.009), 24 hr (P<0.001), 48 hr (P<0.001) (Table 2, Fig. 4). The integration of frequency counted by the computerized device also differed and was higher in group C (52.85±14.66/48 hr) than in group I (24.45±8.06/48 hr)(P<0.001)(Fig. 5).
The outcomes of this study demonstrate the intraperitoneal instillation of ropivacaine to reduce pain significantly after LC. The total fentanyl consumption was reduced in the instillation group in contrast to control (P<0.001). VAS scores were lower in group I than group C during the overall estimated time and as well as the interval times of estimation. During the study no patients were excluded from the study because of uncontrolled pain or undesirable surgical outcomes such as cardiac events, delayed bowel movements and patient intolerance.
Pain is a highly personal experience which is whatever the experiencing person expresses and exist whenever the person appeals. The ambiguity of pain lies in that it is a subjective sensation or emotion and thorough objective observation of such is difficult. Because VAS scores are estimated by patients the accurate measurement is limited and objective estimation of pain could be deleterious, the computerized PCA was supplemented. Proper orientation of the patient to press the button of the PCA device in order to express pain gains objective power in estimating pain. Postoperative pain pleaded by patients who underwent LC was recorded in its frequency by computerized device which differed between the early 24 hours and latter 24 hours of operation. In that latter period pain itself was somewhat recovered the frequency showed statistical difference between the groups which explains the patients' complaint diminishes by the instillation of ropivacaine. The fentanyl consumption velocity reflects the frequency of PCA bolus injection activated by the patient. In our study the FPB showed statistical difference until 24 hours after the operation which implies the 24 hour convalescence period required for recovery.
The limitations of this study were that control group was a sham study group of simple normal saline peritoneal instillation which itself had positive influences on peritoneal irritation and comparison for original uncontrolled pneumoperitoneum omitting priceless comparisons among the groups which might have demonstrated differences in intensity and periods. In this study, intensity of pain was only described on integration of dichotomy, no description of quality but quantity was in concern. Without multifactorial individualized assessment on characters of pain, visceral and somatic pain was indistinguishable in the process. Moreover because the 48 hour limited duration of observation, drawing conclusion of somatic pain relevance which has a delayed onset compared to parietal pain was somewhat restricted.
Previous studies agree that postoperative pain from LC pain consists of 3 components, visceral, parietal, and referred shoulder pain distinguishing from each other in the intensity, latency and duration.(15) Several studies suggested that parietal pain is the predominant cause of pain.(16,17) By contrast, emphasis upon the visceral pain occupying greater portion of pain from LC in the early convalescent period because the surgical manipulation and tissue destruction is the most in the visceral organ itself compared to the small incisions and limited trauma of the abdominal wall.(2,5,10,18)
Multimodal efforts to reduce overall pain and benefit postoperative conditions of patients have been investigated. Reports of injecting local anesthetics on the incision sites in various methods were carried out with anticipation of parietal pain reduction in the injection group, but the results were not concurring which the reason is yet clearly understood. Local anesthesia model showed no significant pain reduction in the LC patients compared to OC group studies.(4,12,19) Furthermore in previous studies, loco-regional anesthetic models showed no apparent effect on the post operative abdominal pain.(4,16) Probably because in these study models, the local injection only plays a role in controlling parietal pain neglecting visceral pain which accounts for the majority of patients' discomfort. The reduction of mere parietal pain was imperceptible to the cholecystectomized patients.
The role of intraperitoneal local analgesic instillation is "preemptive analgesia" which refers that previously administered medications modulate the arousal of nociception action in the post operative period sparing pain-after analgesics. The preemptive analgesia prevents the formation of central sensitization to painful stimuli by decreasing response from pain sensation.(5) As a preemptive method current studies and meta-analysis demonstrates the local anesthesia instillation into the peritoneal space as a safe and effective method in diminishing early post operative pain. In previous studies, ropivacaine showed less cardiotoxicity and central nervous system side effects compared to bupivacaine in same plasma concentration even in large dose (300 mg) of intraperitoneal instillation.(7,20-22)
Although studies vary with timing of instillation and type of medications and yet no credible guideline has been established for the maximal effect, the attractive fact that feasible laparoscopic ropivacaine instillation encompasses significant reduction in postoperative pain and decrement of analgesics without toxicity. In this current study instillation of 2 mg/kg of ropivacaine into the subhepatic space before operation showed significant pain and analgesic reduction on the basis of patients' oriented pain investigation of computerized PCA and VAS score.
LC has evolved since its first operation performed in France 1987 in its technologic instruments and surgical skills. Nowadays speaking of one of the most popular outpatient surgery, LC requires feasible non invasive and simple analgesic procedure when postoperative pain is the most notorious element for shorter convalescence. In conclusion our demonstration of intraperitoneal instillation of ropivacaine can play a momentous role in decreasing patient pain.
Figures and Tables
Fig. 5
Integration comparison in frequency of patient controlled anesthesia (PCA) bolus delivery (*P<0.05).

References
1. Schirmer BD, Edge SB, Dix J, Hyser MJ, Hanks JB, Jones RS. Laparoscopic cholecystectomy. Treatment of choice for symptomatic cholelithiasis. Ann Surg. 1991. 213:665–676.
2. Tsimoyiannis EC, Siakas P, Tassis A, Lekkas ET, Tzourou H, Kambili M. Intraperitoneal normal saline infusion for postoperative pain after laparoscopic cholecystectomy. World J Surg. 1998. 22:824–828.
3. Bisgaard T, Kehlet H, Rosenberg J. Pain and convalescence after laparoscopic cholecystectomy. Eur J Surg. 2001. 167:84–96.
4. Mouton WG, Bessell JR, Otten KT, Maddern GJ. Pain after laparoscopy. Surg Endosc. 1999. 13:445–448.
5. Karaaslan D, Sivaci RG, Akbulut G, Dilek ON. Preemptive analgesia in laparoscopic cholecystectomy: a randomized controlled study. Pain Pract. 2006. 6:237–241.
6. McLauchlan GJ, Macintyre IM. Return to work after laparoscopic cholecystectomy. Br J Surg. 1995. 82:239–241.
7. Barczynski M, Konturek A, Herman RM. Superiority of preemptive analgesia with intraperitoneal instillation of bupivacaine before rather than after the creation of pneumoperitoneum for laparoscopic cholecystectomy: a randomized, double-blind, placebo-controlled study. Surg Endosc. 2006. 20:1088–1093.
8. Wills VL, Hunt DR. Pain after laparoscopic cholecystectomy. Br J Surg. 2000. 87:273–284.
9. Verma GR, Lyngdoh TS, Kaman L, Bala I. Placement of 0.5% bupivacaine-soaked Surgicel in the gallbladder bed is effective for pain after laparoscopic cholecystectomy. Surg Endosc. 2006. 20:1560–1564.
10. Pappas-Gogos G, Tsimogiannis KE, Zikos N, Nikas K, Manataki A, Tsimoyiannis EC. Preincisional and intraperitoneal ropivacaine plus normal saline infusion for postoperative pain relief after laparoscopic cholecystectomy: a randomized double-blind controlled trial. Surg Endosc. 2008. 22:2036–2045.
11. Adams WJ, Avramovic J, Barraclough BH. Wound infiltration with 0.25% bupivacaine not effective for postoperative analgesia after cholecystectomy. Aust N Z J Surg. 1991. 61:626–630.
12. Lepner U, Goroshina J, Samarutel J. Postoperative pain relief after laparoscopic cholecystectomy: a randomised prospective double-blind clinical trial. Scand J Surg. 2003. 92:121–124.
13. Helvacioglu A, Weis R. Operative laparoscopy and postoperative pain relief. Fertil Steril. 1992. 57:548–552.
14. Gupta A, Thorn SE, Axelsson K, Larsson LG, Agren G, Holmstrom B, et al. Postoperative pain relief using intermittent injections of 0.5% ropivacaine through a catheter after laparoscopic cholecystectomy. Anesth Analg. 2002. 95:450–456.
15. Joris J, Thiry E, Paris P, Weerts J, Lamy M. Pain after laparoscopic cholecystectomy: characteristics and effect of intraperitoneal bupivacaine. Anesth Analg. 1995. 81:379–384.
16. Bisgaard T, Klarskov B, Kristiansen VB, Callesen T, Schulze S, Kehlet H, et al. Multi-regional local anesthetic infiltration during laparoscopic cholecystectomy in patients receiving prophylactic multi-modal analgesia: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 1999. 89:1017–1024.
17. Ure BM, Troidl H, Spangenberger W, Dietrich A, Lefering R, Neugebauer E. Pain after laparoscopic cholecystectomy. Intensity and localization of pain and analysis of predictors in preoperative symptoms and intraoperative events. Surg Endosc. 1994. 8:90–96.
18. Papadima A, Lagoudianakis EE, Antonakis P, Filis K, Makri I, Markogiannakis H, et al. Repeated intraperitoneal instillation of levobupivacaine for the management of pain after laparoscopic cholecystectomy. Surgery. 2009. 146:475–482.
19. Kehlet H, Gray AW, Bonnet F, Camu F, Fischer HB, McCloy RF, et al. A procedure-specific systematic review and consensus recommendations for postoperative analgesia following laparoscopic cholecystectomy. Surg Endosc. 2005. 19:1396–1415.
20. Boddy AP, Mehta S, Rhodes M. The effect of intraperitoneal local anesthesia in laparoscopic cholecystectomy: a systematic review and meta-analysis. Anesth Analg. 2006. 103:682–688.
21. Labaille T, Mazoit JX, Paqueron X, Franco D, Benhamou D. The clinical efficacy and pharmacokinetics of intraperitoneal ropivacaine for laparoscopic cholecystectomy. Anesth Analg. 2002. 94:100–105.
22. Knudsen K, Beckman Suurkula M, Blomberg S, Sjovall J, Edvardsson N. Central nervous and cardiovascular effects of i.v. infusions of ropivacaine, bupivacaine and placebo in volunteers. Br J Anaesth. 1997. 78:507–514.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download