Abstract
Purpose
The aim of this study was to find the dose of agonistic 4-1BB monoclonal antibody (mAb) that results in optimal T cell activation.
Methods
Cancer was induced in mice by an intrahepatic parenchymal injection of 1×105 cells of CT26 cells. Cancer-carrying mice (n=84) were divided into seven groups and treated with either rat IgG or agonistic 4-1BB monoclonal antibody (mAb) (5µg, 10µg, 20µg, 100µg, 200µg, or 300µg). All treatments were administered intraperitoneally on days 7, 9, and 11. Mice from each group were sacrificed on days 14, 28, and 42. Harvested livers were weighed and the numbers of T cells in the splenocytes were analyzed with a FACS Vantage flow cytometer.
Results
Liver weights increased when 5µg of agonistic 4-1BB mAb was administered, but showed no additional weight increase for doses greater than 10µg. The absolute numbers of CD4+ and CD8+ T cells increased in groups treated with low doses of agonistic 4-1BB mAb (5µg, 10µg, or 20µg), but did not increase in the groups treated with high doses of mAb (100µg, 200µg, or 300µg). The levels of CD4/annexin V and CD8/annexin V increased as the dose increased, and the absolute cell numbers of CD4/annexin V were greater than those of CD8/annexin V.
Conclusion
Liver weight, including the cancer mass, failed to increase at agonistic 4-1BB mAb doses greater than 10µg. A high dose (≥100µg) of agonistic 4-1BB mAb resulted in lower counts of absolute T cells. This study suggests that a low dose (20µg) of agonistic 4-1BB mAb can be used for optimal T cell activation in combination with other anti-cancer treatments.
The importance of T cell mediated responses for anti-tumor immunity is well known. Optimal activation of T cells for clonal expansion requires at least two distinct biological signals: the interaction of the T cell antigen receptor (TCR) with peptides bound to major histocompatibility complex (MHC) molecules and a functionally defined event called the co-stimulatory pathway.(1) The molecules involved in co-stimulation mostly belong to either the CD28:B7 superfamily or the tumor necrosis factor (TNF) superfamily.(2,3)
CD28:B7 molecules are one of the best studied co-stimulatory pathways, and are thought to comprise the main mechanism underlying primary T cell stimulation. However, a number of other molecules have been identified that amplify and diversify the T cell response following initial T cell activation. These include the more recently described 4-1BB:4-1BB ligand (4-1BBL) molecules.(4)
4-1BB (also called CD137) was first identified in mice in a modified differential screening procedure and is a member of the TNF receptor superfamily of type I membrane protein.(5) It is an inducible protein that is expressed on the surfaces of activated CD4+ and CD8+ T cells,(1,6) activated NK cells,(7) and dendritic cells.(8) The 4-1BB ligand (4-1BBL) is a type II surface glycoprotein of the TNF superfamily. Expression of 4-1BBL is restricted to antigen-presenting cells (APCs) such as dendritic cells, macrophages, and activated B cells.(9)
In vitro, signaling via 4-1BB by agonistic monoclonal antibodies (mAbs) or soluble 4-1BBL or cell lines expressing 4-1BBL in the presence of anti-TCR Abs has been shown to cause T cell expansion, cytokine induction, up-regulation of anti-apoptotic genes, and prevention of activation-induced cell death.(3,10) The in vivo effects of agonistic 4-1BB mAbs are much more dramatic: they promote rejection of cardiac and skin allografts, eradicate established tumors, broaden primary antiviral CD8+ T cell responses, and increase T cell cytolytic potentials.(11-13)
Immunotherapy is an approach to treat intractable diseases such as cancer, autoimmune diseases, transplantation rejection, and chronic infection by enhancing, suppressing, or modifying the immune system.(14) Cancer is thought to have escaped immune surveillance.(4) Cancer immunotherapy aims to generate long-lasting functionally active CD8+ T-cells specific for the tumor cells, and the strategy of cancer immunotherapy has been to inject antibodies that stimulate T cells at target cancer. Administration of agonistic 4-1BB mAbs is an attractive strategy for cancer immunotherapy because 4-1BB signaling promotes T cell and NK cell proliferations and interferon-gamma (IFN-γ) secretion, and prolongs the survival of CD8+ T cells.(15) However, agonistic 4-1BB mAbs also have some effects that are not related to cancer treatment. They accelerate the activation-induced cell death of antigen-specific CD4+ T cells and reduce the incidence and severity of autoimmune disease.
A co-stimulatory signal provided by agonistic 4-1BB mAbs might reinforce the anti-tumor actions of effector cells in vivo. Some investigators reported the anti-tumor effects of agonistic 4-1BB mAbs at low doses (5µg to 100µg), while others reported anti-inflammatory effects at doses greater than 200µg.(16-21)
We analyzed the effects of agonistic 4-1BB mAbs in a murine colon cancer metastasis model. Our aim was to find the dose of agonistic 4-1BB mAb that resulted in optimal T cell activation.
Male eight-week-old Balb/c mice (weight range, 20~25 g) were purchased from the Jackson Laboratory (Bar Harbor, USA). All mice (n=84) were housed under specific pathogen-free conditions and in accordance with institutional guidelines. Cancer was induced by an intrahepatic parenchymal injection of CT26 cells (100 ml, 1×105 cells) (Manassas, ATCC, USA) through a midline skin incision. CT26 cells had initially been induced in a Balb/c mouse by chemical carcinogens, and stable cell lines had been established.(22) Cancer-carrying mice were divided into seven groups of 12 rats each and were treated with rat IgG as a control or with agonistic 4-1BB mAb (Immunomics, Ulsan, Korea) at a dose of 5µg, 10µg, 20µg, 100µg, 200µg, or 300µg. Treatments were administered intraperitoneally on days 7, 9, and 11.
Subsets of four mice from each dosage group were sacrificed on days 14, 28, and 42 after the administration of CT26 cells. Livers and spleens were harvested from the sacrificed mice. Because it was not possible to obtain the weights of the cancer masses alone, liver weights included the cancer masses.
Splenocytes were obtained from the mice through the mechanical dissociation of the spleen followed by lysis of the red blood cells. The surface molecules of T cells were observed in spleens by the use of mAb directed to CD4, CD8, CD11b, CD11c, CD25, CD62, NK cells, and a control isotope conjugated with fluorochromes (FITC, PE). All antibodies were purchased from BD Pharmingen (San Diego, CA, USA), and sample analyses were carried out on a FACS Vantage flow cytometer (BD Bioscience, San Jose, CA, USA). Absolute cell numbers were calculated by multiplying the flow cytometry percentages by the total numbers of viable cells.
Annexin V was used to quantitate the percentage of cells within a population that were actively undergoing apoptosis. 7-Amino-actinomycin (7-AAD) is a standard flow cytometric viability probe and was used in this study to distinguish viable cells from non-viable cells. Cells that stained positive for annexin V and negative for 7-AAD were undergoing apoptosis. Briefly, cells were washed twice with cold phosphate buffered saline (PBS) and resuspended in binding buffer (annexin V-PE kit; BD Biosciences, USA). One hundred microliters of the binding buffer was transferred to a 5 ml culture tube followed by the addition of 5µl of annexin V-PE and 5µl of 7-AAD. After a 15 min incubation period at room temperature in the dark, 400µl of binding buffer was added. In CD4+ and CD8+ T cells, apoptosis was observed through the use of mAbs directed against CD4+, CD8+, and the fluorochromes (FITC)-conjugated control isotype.
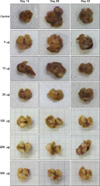
To investigate the role of 4-1BB the therapeutic effect of agonistic 4-1BB mAb in an experimental cancer model, cancer was induced in Balb/c mice. Liver size changed with an increasing dose of agonistic 4-1BB mAb and over time (Fig. 1). Gross examination of the liver size revealed no increase when more than 20µg of agonistic 4-1BB mAb was used. However, an increase in liver size was seen for the control and at dose of 5µg and 10µg of agonistic 4-1BB mAb. The liver weight increased when 5µg of agonistic 4-1BB mAb was given, but did not increase at doses larger than 10µg (P<0.05). In other words, 5µg of agonistic 4-1BB mAb failed to show significant tumor suppression, whereas higher doses of agonistic 4-1BB mAb (10µg to 300µg) suppressed tumor growth and increase in liver weight to similar degree (Fig. 2).
Absolute cell numbers of CD4+ and CD8+ T cells increased over time in the low dose agonistic 4-1BB mAbs treatment groups (5µg, 10µg, 20µg, P<0.05), but not in the high dose groups (100µg, 200µg, 300µg) (Fig. 3, 4). These numbers were higher at 42 days than at 28 days in the low dose groups (10µg and 20µg; P<0.05). The absolute numbers of CD4+ T cells peaked at 28 days in the high dose groups (100µg and 200µg). This peak level was lower than that of the low dose group at 42 days (P<0.05). The absolute numbers of CD8+ T cells peaked at day 14 days for the 100µg dose group, whereas they peaked at day 28 and then decreased for the high dose groups (200µg and 300µg). Similar patterns of change in CD4+ and CD8+ T cells were observed for CD62L-low T cells, CD4/CD25 T cells, and IFN-γ producing cells (Fig. 3, 4).
CD4/annexin V and CD8/annexin V peaked at 28 days in the high dose groups (100µg, 200µg, or 300µg of agonistic 4-1BB mAb) (Fig. 5). The absolute cell number for a dose of 300µg agonistic 4-1BB mAb was higher than that for the 100µg dose treatment (P<0.05). The absolute number of CD4/annexin V cells was greater than that of CD8/annexin V cells (P<0.05). The absolute cell number for each dose decreased at 42 days (Fig. 5).
Patterns of change in CD11c and natural killer (NK) cells were comparable to those of the T cells (Fig. 6). The absolute cell numbers increased for the low dose agonistic 4-1BB mAb groups (5µg, 10µg, 20µg), but not for the high dose groups (100µg, 200µg, 300µg). However, for CD11b cells, the absolute cell numbers for the low dose groups (5µg, 10µg, 20µg) peaked at 28 days and decreased at 42 days (Fig. 6A). The absolute cell numbers for the high dose group (100µg, 200µg, 300µg) were all lower than those for the low dose group (Fig. 6).
The physiology and geography of T cell activation require the capture of tumor antigens by antigen-presenting cells (APCs) at the tumor site. After processing, tumor antigens are expressed on the surfaces of antigen-presenting cells in the form of MHC class I or II peptide complexes. Antigen-presenting cells migrate to secondary lymphoid organs where tumor antigens are specifically recognized by naïve T cells. T cell receptor (TCR) engagement delivers the first activation signal that induces the cell-surface expression of co-stimulatory molecules. These molecules then deliver the second activation signal that leads to the full activation of T cells. Most T cells are activated in secondary lymphoid organs.
The sequence of events accounting for the mechanism of action of agonistic 4-1BB mAbs in anti-tumor therapy was originally postulated as follows: tumor antigens bind to TCRs and stimulate T cells to up-regulate 4-1BB.(12) Systemic administration of agonistic 4-1BB mAb co-stimulates the T cells that have up-regulated 4-1BB, and 4-1BB then delivers signals that both prevent apoptosis and promote effector functions of anti-tumor cytotoxic T lymphocytes.(1)
Shuford et al.(11) demonstrated that signaling through 4-1BB preferentially induces proliferation of CD8+ T cells. Agonistic 4-1BB mAb also prevents superantigen-induced cell death in CD8+ T cells and significantly increases the survival of CD8+ T cells in vivo.(23)
The anti-tumor effect of agonistic 4-1BB mAbs mainly depends on increased tumor-specific cytolytic T lymphocyte activity and IFN-γ production by CD8+ T cells, as well as an anti-apoptosis effect in CD8+ cytolytic T lymphocytes.(15) This anti-apoptotic effect extends the operative life of CD8+ T cells and reverses the cytotoxic T lymphocyte established anergy in vivo.(24)
We studied T cells in mice that were of the same age (eight weeks), gender (male) and genetic background (Balb/c from Jackson laboratory) and were thus considered equivalent prior to treatment.
The absolute numbers of CD8+ T cells were influenced by agonistic 4-1BB mAbs over time. These numbers increased in the low dose (≤20µg) groups, but decreased in the high dose (≥100µg) groups. Other studies have shown that 5µg of agonistic 4-1BB mAb promotes survival of CD8+ T cells and enhances CD8+ T cell expansion.(25,26) We found that the absolute cell number of IFN-γ producing CD8 T cells peaked on day 42 when 5µg agonistic 4-1BB mAb was used. However, the similar patterns of absolute cell numbers in the control and 5µg treatment groups may be attributed to the cancer itself. The absolute numbers of CD8+ T cells decreased after a peak at 14 days of treatment with 100µg of agonistic 4-1BB mAbs, whereas for groups receiving 200µg and 300µg of mAb, these numbers peaked at 28 days. At 14 days, the absolute cell numbers of the high dose (≥100µg) groups were higher than those of the low dose (≤20µg) groups. Therefore, the abrupt increase in T cells numbers caused by a dose of agonistic 4-1BB mAb greater than 100µg caused activation-induced cell death, resulting in a sudden decrease in absolute cell numbers by days 28 and 42. The peak level of CD8/annexin V became higher with increases in the dose of agonistic 4-1BB mAb, meaning there was less apoptotic activity associated with low doses of agonistic 4-1BB mAb.
Signaling through 4-1BB appears to promote cell proliferation and survival in vitro,(27) but the mechanism of 4-1BB co-stimulation in CD4+ T cells differs from that in CD8+ T cells.(20) 4-1BB-mediated signals were associated with CD4+ T cell responses in vivo, including induction of T helper cell anergy, an alloimmune response, acute inflammation, an autoimmune disease and a type 1 T helper (Th1) cell response to tumor cells.(20,28)
This study showed that the absolute count of CD4+ T cells increased with low mAb doses (≤20µg) at 42 days, but was maintained or decreased with a high doses (≥100µg). The absolute cell numbers of the high dose treatment groups (100µg, 200µg, and 300µg) peaked at 28 days and decreased by day 42. The decrease in the number of CD4+ T cells may have been more pronounced due to activation-induced cell death.
We used rat IgG as the negative control in this study. Unlike the specific effects of agonistic 4-1BB mAb, IgG does not affect the immune system. IgG alone cannot induce an increase in T cells; however, the increases in absolute cell number of CD4 and CD8 T cells may be a side effect of the cancer or of the high dose of agonistic 4-1BB mAb, which could result in splenomegaly in mice. In addition, despite the use of mice that were as similar as possible, cell numbers may vary due to the natural variation encountered in in vivo studies.
Although co-stimulatory signaling via 4-1BB induces clonal expression of CD4+ T cells shortly after immunization, the activated CD4+ T cells are rapidly cleared by activation-induced cell death.(20) Therefore, the administration of agonistic 4-1BB mAb may provide a new immunotherapeutic approach in treating CD4+ T cell-mediated disease. A high dose (≥100µg) of agonistic 4-1BB mAb may be particularly useful in treating Th2-mediated autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, and ulcerative colitis.
The patterns of change of the absolute cell numbers of CD11c and NK cells were similar to those of CD4+ and CD8+ T cells. Innate immune cells such as CD11c and NK cells were increased the administration of a low dose of agonistic 4-1BB mAb and were inhibited by a high dose of mAb. However, the absolute cell number of CD11b first increased and then decreased by day 42. These patterns may be due to activation-induced cell death as absolute cell numbers of T cells decreased in the high dose treatment group (≥100µg).
The administration of agonistic 4-1BB mAbs leads to regressions of established B10.2 fibrosarcoma,(17) myeloma,(18) and CD 26 colon carcinoma.(21) These antitumor effects were achieved with an agonistic 4-1BB mAb dose of 100µg. Our study suggests that a low dose (20µg) could have even better results.
A high dose of agonistic 4-1BB mAb (200µg) results in amelioration of autoimmune encephalomyelitis, rheumatoid arthritis, and inhibition of chronic graft versus host disease.(16,19,20) Similarly, our study suggests that better results may be obtained with the use of an even higher dose of agonistic 4-1BB mAb (300µg).
In addition to mono-therapy, most recent studies have involved the administration of agonistic 4-1BB mAb as adjuvant treatment in combination with other therapeutic approaches. For example, agonistic 4-1BB mAb was administered as a component in combined treatment of cancer with chemotherapy,(29) IL-12 therapy,(30) or with other immunomodulatory antibodies.(31) Agonistic 4-1BB mAb has also been used in conjunction with different monoclonal antibodies against other co-stimulatory molecules.
The objective of this study was to find a dose of agonistic 4-1BB mAb that results in optimal T cell activation in a mouse cancer model. The weight of the liver, including the cancer mass, increased with either control treatment or 5µg of agonistic 4-1BB mAb, but it showed no increase at doses larger than 10µg. The absolute numbers of CD4+ and CD8+ T cells increased with time for the low dose (20µg) treatment with agonistic 4-1BB mAb, but did not increase at high doses (≥100µg). More apoptotic activity occurred when a high dose (≥100µg) of agonistic 4-1BB monoclonal antibodies was used. This study suggests that a low dose (20µg) of agonistic 4-1BB mAb can be used for optimal T cell activation in combination with other anti-cancer treatments.
Figures and Tables
 | Fig. 2Liver weights and doses of agonistic 4-1BB monoclonal antibodies. Weight increased for the control and with 5µg of agonistic 4-1BB mAb, but showed no increase at doses ≥10µg. The absolute cell numbers of the control and the 5µg dose group significantly increased over time (*P<0.05). |
 | Fig. 3(A) CD4, (B) CD4/CD62L low cell population, and (C) CD4/CD25 cell population. The absolute cell numbers increased in the low dose (≤20µg) groups, but not in the high dose groups (≥100µg). Absolute cell numbers peaked on day 28 in the high dose groups and decreased by day 42. In contrast, the absolute cell numbers on day 42 were significantly higher than those at day 14 for doses of agonistic 4-1BB mAb (≤20µg; *P<0.05). |
 | Fig. 4(A) CD8, (B) CD8/CD62L low cells population, and (C) interferon-gamma (IFN-γ) producing CD8 cell population. The absolute cell numbers of CD8 T cells increased with 5µg of agonistic 4-1BB mAb, but did not increase with 10 µg or 20µg treatments. The absolute cell number of CD8 T cell for 100µg of mAb peaked on day 14, decreased on day 28 and decreased further on day 42 (P<0.05). However, absolute cell numbers of T cells peaked at day 28 for 200µg and 300µg mAb. The absolute cell numbers of IFN-γ producing CD8 T cells gradually decreased over time at high doses of agonistic 4-1BB mAb (≥100µg; P<0.05). |
 | Fig. 5Apoptotic cell proportion. (A) CD4/annexcin V, (B) CD8/annexin V. CD4/annexin V and CD8/annexin V levels in the high dose groups (100µg, 200µg, 300µg of agonistic 4-1BB mAb) peaked on day 28. The absolute cell number for 300µg mAb was higher than that for 100µg (P<0.05). The absolute number of CD4/annexin V cells was greater than that of CD8/annexin V cells (P<0.05). |
References
1. Vinay DS, Cha K, Kwon BS. Dual immunoregulatory pathways of 4-1BB signaling. J Mol Med. 2006. 84:726–736.
2. Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005. 23:515–548.
3. Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005. 23:23–68.
4. Cheuk AT, Mufti GJ, Guinn BA. Role of 4-1BB:4-1BB ligand in cancer immunotherapy. Cancer Gene Ther. 2004. 11:215–226.
5. Kwon BS, Kozak CA, Kim KK, Pickard RT. Genomic organization and chromosomal localization of the T-cell antigen 4-1BB. J Immunol. 1994. 152:2256–2262.
6. Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, et al. Inducible T cell antigen 4-1BB. Analysis of expression and function. J Immunol. 1993. 150:771–781.
7. Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998. 190:167–172.
8. Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002. 14:275–286.
9. Laderach D, Movassagh M, Johnson A, Mittler RS, Galy A. 4-1BB co-stimulation enhances human CD8(+) T cell priming by augmenting the proliferation and survival of effector CD8 (+) T cells. Int Immunol. 2002. 14:1155–1167.
10. Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003. 3:609–620.
11. Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997. 186:47–55.
12. Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997. 3:682–685.
13. Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol. 2002. 3:536–541.
14. Choi BK, Kim YH, Kang WJ, Lee SK, Kim KH, Shin SM, et al. Mechanisms involved in synergistic anticancer immunity of anti-4-1BB and anti-CD4 therapy. Cancer Res. 2007. 67:8891–8899.
15. Sun Y, Chen JH, Fu Y. Immunotherapy with agonistic anti-CD137: two sides of a coin. Cell Mol Immunol. 2004. 1:31–36.
16. Kim J, Choi WS, La S, Suh JH, Kim BS, Cho HR, et al. Stimulation with 4-1BB (CD137) inhibits chronic graft-versus-host disease by inducing activation-induced cell death of donor CD4+ T cells. Blood. 2005. 105:2206–2213.
17. Miller RE, Jones J, Le T, Whitmore J, Boiani N, Gliniak B, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002. 169:1792–1800.
18. Murillo O, Arina A, Hervas-Stubbs S, Gupta A, McCluskey B, Dubrot J, et al. Therapeutic antitumor efficacy of anti-CD137 agonistic monoclonal antibody in mouse models of myeloma. Clin Cancer Res. 2008. 14:6895–6906.
19. Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med. 2004. 10:1088–1094.
20. Sun Y, Chen HM, Subudhi SK, Chen J, Koka R, Chen L, et al. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat Med. 2002. 8:1405–1413.
21. Taraban VY, Rowley TF, O'Brien L, Chan HT, Haswell LE, Green MH, et al. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur J Immunol. 2002. 32:3617–3627.
22. Corbett TH, Griswold DP Jr, Roberts BJ, Peckham JC, Schabel FM Jr. Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975. 35:2434–2439.
23. Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J Immunol. 1999. 162:5037–5040.
24. Wilcox RA, Tamada K, Flies DB, Zhu G, Chapoval AI, Blazar BR, et al. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood. 2004. 103:177–184.
25. Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002. 169:4882–4888.
26. Lee HW, Nam KO, Park SJ, Kwon BS. 4-1BB enhances CD8+ T cell expansion by regulating cell cycle progression through changes in expression of cyclins D and E and cyclin-dependent kinase inhibitor p27kip1. Eur J Immunol. 2003. 33:2133–2141.
27. Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001. 167:1313–1324.
28. Kwon B, Lee HW, Kwon BS. New insights into the role of 4-1BB in immune responses: beyond CD8+ T cells. Trends Immunol. 2002. 23:378–380.
29. Kim YH, Choi BK, Kim KH, Kang SW, Kwon BS. Combination therapy with cisplatin and anti-4-1BB: synergistic anticancer effects and amelioration of cisplatin-induced nephrotoxicity. Cancer Res. 2008. 68:7264–7269.
30. Li Q, Pan PY, Gu P, Xu D, Chen SH. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 2004. 64:1130–1139.
31. Gray JC, French RR, James S, Al-Shamkhani A, Johnson PW, Glennie MJ. Optimising anti-tumour CD8 T-cell responses using combinations of immunomodulatory antibodies. Eur J Immunol. 2008. 38:2499–2511.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download