Abstract
Intracystic papillary carcinoma (IPC) is an extremely rare disease in the male breast with a few case reports. We present a case of a 61-year-old male who had IPC and review regarding diagnosis, characteristics and treatment. He had a chief complaint of a subareolar mass. It was diagnosed as a benign cystic intraductal papilloma by fine needle aspiration outside hospital. His radiologic studies including mammography and ultrasonography showed a suspicious malignant mass categorized as a BIRADS 4A in the right subareolar area. Therefore, the patient underwent wide excision without sentinel lymph node biopsy. The final pathologic results revealed a 1.6 cm sized intraductal papillary carcinoma of low nuclear grade with clear resection margin. He has taken tamoxifen and received adjuvant radiation therapy.
The breast cancer in male is uncommon with the incidence of 0.6% of all breast carcinomas and less than 1% of all malignancies in men.(1) Among the breast cancer, intracystic papillary carcinoma (IPC) is rarer, accounting for 0.5~1% of all breast cancer with a few case reports about IPC in male breast cancer to date. It typically occurs at an old age with a good prognosis.(2) One study reported 10-year survival rate for IPC is 100%, the recurrence-free survival rate is 96% and 77% at 2 and 10 years, respectively.(3) We report the case of IPC occurred in right breast of a 61-year-old male with review of IPC regarding diagnosis, characteristics, and treatment.
A 61-year-old male was admitted for the abnormal palpable bean sized movable mass in his right breast. He had a chief complaint of a subareolar mass without nipple discharge for almost 1 year. At an outside hospital, he underwent ultrasonography guided fine needle aspiration (FNA); pathologic result revealed that there are papillary ductal cell nests and hemosiderin-laden macrophages, consistent finding with cystic intraductal papilloma. He is a healthy hepatitis B virus carrier, taking a medication due to benign prostate hypertrophy. He underwent excision of the left foot due to pyogenic granuloma 12 years ago. There was no other medical problem. There was no trauma history to the chest or familial history of malignancy including breast cancer. On physical examination, we palpated a 1.5 cm sized, well marginated, round, movable mass in right breast lower center, subareolar area. Mammography revealed a 1.8 cm suspicious malignant mass in right subareolar area (Fig. 1). Ultrasonography showed that about 1.6 cm septated cystic mass with some solid portion, without increased vascularity is in his right subareolar area (Fig. 2). It was categorized as a BIRADS 4A (low suspicion of malignancy) lesion. The patient proceeded to wide excision including normal breast tissue to gain a safety margin for the fear of risk of malignancy sparing nipple-areolar complex. We cut the specimen; it was cystic mass containing brownish clear fluid (Fig. 3). The final pathologic examination revealed a 1.6 cm sized intraductal papillary carcinoma of low nuclear grade, no endolymphatic tumor emboli with clear resection margin (Fig. 4). It had low nuclear grade without necrosis, 1/3 of Van Nuys classification, and no stromal invasion. Immunohistochemistry demonstrated estrogen receptor and progesterone receptor positivity and HER-2 negativity. The patient has taken 20 mg tamoxifen once a day as an adjuvant therapy, and received adjuvant radiation therapy of 50 Gy, 25 fractions.
Male breast cancer is uncommon with the incidence of 1/100,000 persons approximately. Male breast cancer somewhat differs from female breast cancer. More than 90% of male breast cancer is ductal carcinoma rather than lobular carcinoma, estrogen receptor-positive tumors are more common, and it occurs at an older age than female breast cancer.(4) The prognosis is poor as compared with female breast cancer due to higher stage; causes are rarity of disease, later identification, and progress of disease such as invasion to skin, chest wall rather than intrinsically aggressive nature. IPC in male breast has a relatively higher incidence of 5~7.5% than females.(5)
Symptoms are mainly painless, non-tender, palpable mass in subareolar area. Diagnosis is made by mammography, ultrasonography, and FNA or core needle biopsy. The mammographic finding of IPC is non-specific, well-marginated mass or negative on small lesion. Sonographically, it usually has a well circumscribed, septated cystic mass including irregular solid, hypoechoic portion.(6,7) FNA cytology or core needle biopsy is important tools to get an accurate diagnose of IPC; some studies reported that IPC is mostly diagnosed with core needle biopsy.(8) However, it cannot always differentiate from benign and malignancy. As a result, an excisional biopsy may be needed to make a proper diagnosis for the suspicious lesion.
The IPC is divided into three subgroups: pure IPC, IPC with associated DCIS, and IPC with associated invasive cancer. Solorzano et al.(3) suggested standard treatment of IPC should be based on associated pathology. There is no definite guideline on the treatment of IPC, however, radical surgical excision with clear resection margin is important mainstay of treatment. Mastectomy is usually unessential, unless it is required, because the IPC has an excellent prognosis, low local recurrence, and rare distant metastasis.(7,9) In other study, all 77 patients with IPC were alive, metastases occurred only in 4% of patients during 10 year follow up.(10) Routine axillary dissection is not recommended because one of the clinical characteristics of IPC is low incidence of axillary lymph node metastasis. However, sentinel lymph node biopsy in IPC associated with invasion is seemed to be justified as an alternative procedure to assess axillary lymph node status and avoid unnecessary full axillary dissection. The impact of adjuvant hormonal treatment, radiotherapy is still controversial according to the reports. The further studies about consensus regarding appropriate treatment of the IPC are needed hereafter.
Figures and Tables
Fig. 1
Mammography demonstrated about 1.8 cm sized suspicious malignant mass in right subareolar area with BIRADS 4A category (low suspicious malignancy) and recommended pathologic confirmation.

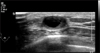
Fig. 2
Ultrasonography demonstrated about 1.6 cm septated cystic mass revealed intraductal papilloma by outside FNA in right subareolar area with BIRADS 4A category.
References
1. Tan PH, Sng IT. Male breast cancer: a retrospective study with immunohistochemical analysis of hormone receptor expression. Pathology. 1997. 29:2–6.
2. Romics L Jr, O'Brien ME, Relihan N, O'Connell F, Redmond HP. Intracystic papillary carcinoma in a male as a rare presentation of breast cancer: a case report and literature review. J Med Case Reports. 2009. 3:13.
3. Solorzano CC, Middleton LP, Hunt KK, Mirza N, Meric F, Kuerer HM, et al. Treatment and outcome of patients with intracystic papillary carcinoma of the breast. Am J Surg. 2002. 184:364–368.
4. Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006. 367:595–604.
5. Dragoumis DM, Tsiftsoglou AP. Intracystic papillary carcinoma associated with ductal carcinoma in situ in a male breast. J Postgrad Med. 2008. 54:39–40.
6. Sinha S, Hughes RG, Ryley NG. Papillary carcinoma in a male breast cyst: a diagnostic challenge. Ann R Coll Surg Engl. 2006. 88:W3–W5.
7. Kinoshita T, Fukutomi T, Iwamoto E, Takasugi M, Akashi-Tanaka S, Hasegawa T. Intracystic papillary carcinoma of the breast in a male patient diagnosed by core needle biopsy: a case report. Breast. 2005. 14:322–324.
8. Carter D, Orr SL, Merino MJ. Intracystic papillary carcinoma of the breast. After mastectomy, radiotherapy or excisional biopsy alone. Cancer. 1983. 52:14–19.
9. Grabowski J, Salzstein SL, Sadler GR, Blair S. Intracystic papillary carcinoma: a review of 917 cases. Cancer. 2008. 113:916–920.
10. Lefkowitz M, Lefkowitz W, Wargotz ES. Intraductal (intracystic) papillary carcinoma of the breast and its variants: a clinicopathological study of 77 cases. Hum Pathol. 1994. 25:802–809.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download