Abstract
Purpose
The platinum-based modified FOLFOX-6 has been reported as an acceptable chemotherapeutic regimen in treatment of advanced gastric cancer patients. The response rate and drug-induced toxicity of platinum-based chemoagents is different according to several gene polymorphism such as ERCC1, XRCC1 and GSTP1 genes, which were related with therapeutic mechanisms. We aimed to evaluate the effect of gene polymorphism and determine the possibility as prediction factor for responsibility in advanced and recurrent gastric cancer patients treated with modified FOLFOX-6 regimen.
Methods
This study was conducted with 55 patients. We sampled 20 ml of peripheral blood to isolate DNA from lymphocytes, and identified genotypes of 3 genes (ERCC1, XRCC1, GSTP1) by PCR-RFL of extracted DNA. Based on medical records, retrospective analysis was made on the patients' clinical characteristics.
Results
The overall response rate to modified FOLFOX-6 was 40.0% (22/55). In polymorphism of ERCC1 C8092A, the wild type (CC) showed a statistically significantly lower response rates to chemoagents than the mutant type (CA/AA). In the subtypes of ERCC1 C118T, however, the wild type (CC) showed statistically significantly lower hematological toxicity than the mutant type (CA/AA). But, there was no statistically significance in survival analysis.
Gastric cancer is the common cancer, worldwide. Recently the incidence of early gastric cancer has increased compared to that of advanced gastric cancer because the advances in diagnostic tools, and have made it possible to detect in early stage. But, more advanced gastric cancer also has increased relatively at the time of diagnosis.(1) In treatment of advanced gastric cancer patients, the platinum-based chemoagents has been usually used as the choice of drug. Especially, modified FOLFOX-6 (combined regimen which is composed of oxaliplatin, 5-Fluorouracil [5-FU] and folinic acid) has been reported as acceptable chemotherapeutic regimen that the average response rate is reported 38~56% in previous literatures.(2-4)
However, the response rate of chemoagents and drug-induced toxicity is revealed difference as patients and duration of chemotherapy. Consequently, the selection of patients group which is expected with possibility for high responsibility and low adverse effect is the most important factor for increasing of chemotherapeutic effect.
The therapeutic activity of injected chemoagents is influenced by numerous factors in human body. It has been thought that the polymorphism of genes, which expressed protein related with therapeutic mechanism, will be a key role in drug activity and the resistance for chemoagents. Finally, the therapeutic response rate and drug-induced toxicity will be changed differently to expected pharmacologic effect.(5)
This study is aimed to evaluate the effect of gene polymorphism of ERCC1 (Excision repair cross complementation group 1), XRCC1 (X-ray repair cross complementation group 1) and GSTP1 (Glutathione S-transferase P1) genes, which were related with therapeutic mechanism of platinum-based chemoagents, about therapeutic response rate and drug-induce toxicity in advanced and recurrent gastric cancer patients treated with modified FOLFOX-6 regimen.(6-8) As well as, we aimed to determine the possibility as prediction factor for responsibility.
This study was conducted with 55 patients for whom peripheral blood sampling before the administration of chemoagents and the response evaluation were possible after chemotherapy, who were selected from total 82 unresectable highly advanced or recurrent gastric cancer patients who had been diagnosed in Seoul St. Mary's Hospital during the period from March 2006 to December 2007, and treated by modified FOLFOX-6 as the primary chemotherapy. The enrolled criteria were; 1) patients with pathologically confirmed gastric cancer, 2) patients without any history of previous chemotherapy except adjuvant chemotherapy, 3) patents whose WHO performance status was 0~2 points and 4) patients with white blood cell count ≥4,000 cells, hemoglobin ≥9.5 g/dl, platelet ≥ 100,000/L, AST & ALT <30 U/L, creatinine <1.5 mg/dl and bilirubin <1.5 mg/dl.
According to modified FOLFOX-6 regimen, the all patients were treated with oxaliplatin (100 mg/m2) and folic acid (100 mg/m2) for 2 hours, and then injected with 5-FU (2,400 mg/m2) continuously for 46 hours at intervals of 2 weeks. The chemotherapy was planned at least 3 cycles for the first response evaluation, but it was stopped when toxicity was severe or the patient refused to continue the chemotherapy.
The response evaluation was made regularly every 6 weeks according to the Response Evaluation Criteria in Solid Tumors (RECIST criteria ver. 1.1). The target lesions recorded and measured at baseline CT. Most of measured target lesions were pathologic lymph node which were met the criterion of a short axis of ≥15 mm by CT scan. If there were identified new lesions in solid organ, these lesions were included in target lesion. The response group included both complete response and partial response, and the no-response group included stable diseases and progressive diseases.
Toxicity was classified according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC version 3.0), and the patients were divided into Grade 1 and 2, and Grade 3 and 4 according to the level of the major toxicities of oxaliplatin, which are neuropathy, hematologic toxicities and mucositis.
As to specimens, 20 ml of peripheral whole blood was sampled before the application of chemotherapy, and DNA was isolation from lymphocytes using the DNA extraction kit (QIAamp® DNA Mini kit, QIAGEN Inc., Hilden, Germany). In the DNA isolation process from 57 blood samples, 2 inadequate samples were excluded from the case. We identified genotypes of 3 genes (ERCC1, XRCC1, GSTP1) by PCR-RFL (polylmerase chain reaction-restriction fragment length) of extracted DNA (Fig. 1). The PCR conditions, products, restriction enzymes and genotypes are showed in Table 1.(9-11)
The clinical data which were consisted with the patients' baseline characteristics, chemotherapy response, toxicity level, disease duration, and overall survival according to the genotypes of gene polymorphism was obtained based on prospectively collected medical records during follow up periods. Overall survival time was calculated from the date of diagnosis to the date of last follow-up or death from any cause. All subjects signed a written informed consent, and the data collection followed the guidelines of the Ethical Committee of the College of Medicine, The Catholic University of Korea.
The analysis was performed according to each gene genotypes. The age factor was divided into 2 groups (standard point: 65 year old) as categorical scale. The cross tabulation method with chi-square or Fisher's exact test was used as univariate analysis, and survival analysis was performed using Kaplan-Meier methods with log-rank test. The P-value was set for below 0.05 for statistical significance. The SAS (ver. 8.2) was used as analysis tool.
The patients' age ranged between 33 and 74 years old, and the men-women ratio was 2.6:1 (41 males and 16 females). Sectional 55 cases that enrolled in this study were performed modified FOLFOX-6 more than 3 cycles at least, and the median value was 8 cycles (range: 3 to 21 cycles). The overall response rate to modified FOLFOX-6 was 40.0% (22/55). The median progression-free survival was 5 months, and the median overall survival was 14 months.
The gene expression rate according to each gene is shown in Table 2. The mutant type (56.4%) of ERCC1 C8092A was higher rate than wild type (43.6%) differently to other genes.
There was no significant difference in age, sex, and pathologic stage according to the subtype of gene polymorphism. In aspect of response rate, the wild type (CC) showed only a statistically significantly lower response rate to chemoagents than the mutant type (CA/AA) in polymorphism of ERCC1 C8092A (P=0.046) (Table 3.) In comparison of overall survival between the wild and the mutant type in polymorphism of ERCC1 C8092A, there was no statistically significance (Fig. 2).
In comparison of toxicity among the phenotypes, no significant difference was observed in toxicity among XRCC1, GSTP1, and ERCC1 C8092A. In the subtypes of ERCC1 C118T (codon118), however, the wild type (CC) showed statistically significantly lower hematological toxicity than the mutant type (CT/TT) (P=0.029) (Table 4).
For highly advanced gastric cancer, various chemoagents have been used through single regimen or multiple combined regimens. The 5-FU was developed firstly in 1957 and has been used until now in combined regimens with other drugs. Later, platinum-based chemoagents such as Cisplatin or Oxaliplatin were developed and have been used with 5-FU. Oxaliplatin is a third generation platinum-based agent, and its therapeutic mechanism is inducing damage by forming complex material with DNA base, consequently, blocking DNA duplication and inhibiting the growth and proliferation of cancer cells (6,12). Among the numerous chemotherapy regimens, modified FOLFOX-6 regimen is reported to show an overall response rate of 38~56% in advanced gastric cancer.(2-4) In our study as well, the overall response rate in the unresectable highly advanced gastric cancer group was 40.0%. However, the response rate of chemotherapy varies among individuals, and no factor has been identified to predict its effectiveness. Indeed, Interindividual variability in drug efficacy has multiple sources. The therapeutic activities of such chemoagents are related with the restoration of damaged DNA and cellular detoxification for the chemoagents. However, the concentration of chemoagents could be controlled by the change of intracellular transport. They show difference in the level of in-vivo activity, and the response or toxicity appears differently among individuals according to difference in activity.(13,14)
In general, DNA damaged by various causes is repaired through mechanisms such as nucleotide excision repair (NER), base excision repair (BER), mismatch repair (MMR), double-strand break repair, and direct repair pathway.(6) Among them, NER, MMR and BER are known to be related with the repair mechanism of damaged DNA by platinum-based chemoagents, and these repair systems arouses resistance and decrease the therapeutic effect of the chemoagents. Among the 3 mechanisms, NER and BER are more closely related with oxaliplatin, and MMR is reported to be related mainly with cisplatin and carboplatin.(6,15)
The ERCC1 protein plays an important role in NER process by removing damaged DNA. The NER pathway has been also considered as major pathway in resistance to oxaliplatin-based chemotherapy. The DNA repair process through NER pathway begins with checking the damaged portion, the double helical structure is unraveled and the damaged portions were removed. Finally, the DNA base sequence is restored from damage induced from chemoagents. Consequently, the elevation of ERCC1 protein is restored DNA which was damaged by oxaliplatin, and then make resistance to oxaliplatin and the DNA duplication of cancer cells is activated and progress more aggressively.(6,13) However, there are 2 common polymorphisms in ERCC1. One is that C is altered to T as single nucleotide polymorphism (SNP) occurs in codon 118 (ERCC1 C118T CT or TT), and related with mRNA level of ERCC1 gene. The other is that C is changed to A as SNP occurs in 8092 nucleotide of 3'-UTR portion (ERCC1 C8092A CA or AA), and related with mRNA stability of ERCC1.(9) It was reported that when the mRNA level is increased the response rate to chemoagents becomes decreased, and when it is decreased the response rate is increased in previous studies.(16-18) In our results, the response rate to modified FOLFOX-6 regimen was significantly higher in the wild type (CC) than in the mutant type (CA/AA) of ERCC1 C8092A, and the ERCC1 C118T wild type (CC) revealed lower hematological toxicity than mutant type (CT/TT). But, there was no significant difference for disease free and overall survival. It means that patients who expressed C8092A or codon 118 wild type of ERCC1 gene can be just tolerable during chemotherapy without prediction of good survival. However, that is more meaningful in aspect of quality of life (QOL) of gastric cancer patients.
The BER pathway is started from detection of the site of defected single strand, and repair of the nucleotides. XRCC1 protein plays an important role in BER process, and gene polymorphism is SNP in which G is changed to A in codon 399 (codon 399 GA or AA).(10) However, there was clinically no meaning in our results.
In addition, several enzymes involved in the metabolism of drug are also believed to affect the therapeutic activity of chemoagents, and among such metabolism-related enzyme. The glutathione S-transferase P1 (GSTP1), which reduces the toxicity of platinum-based chemoagents, is presumed to play an important role. It is believed that SNP in codon 105 of the GSTP1 gene, in which A is changed to G (GSTP1 codon 105 AG), is related with the activity of metabolic enzyme and is an important form of causing difference in the therapeutic effect and toxicity of platinum-based chemotherapy.(11,19) But, it was not also clinically meaningful gene polymorphism in our study.
This study has some limitations. Although the analysis included evaluation of 4 genes at one setting, a relatively small number of cases were obtained. However, this outcome can be considered as preliminary study for further large-scale study.
In conclusion, we suggest that ERCC1 gene polymorphism is clinically more adequate as a feasible factor for predicting the response rate and toxicity of modified FOLFOX-6 regimen in gastric cancer patients, and a larger-scale study would be considerate for definitive evidence.
Figures and Tables
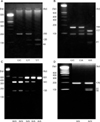 | Fig. 1PCR-RFL (polylmerase chain reaction-restriction fragment length) results of polymorphism according to genes. (A) ERCC1 C118T, (B) ERCC1 C8092A, (C) XRCC1, (D) GSTP1. |
References
1. Ministry of Health and Welfare. 2002 Annual Report of the Korea Central Cancer Registry: Based on Registered Data from 139 Hospitals. 2003. Seoul: Ministry of Health and Welfare.
2. Louvet C, Andre T, Tigaud JM, Gamelin E, Douillard JY, Brunet R, et al. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol. 2002. 20:4543–4548.
3. Al-Batran SE, Atmaca A, Hegewisch-Becker S, Jaeger D, Hahnfeld S, Rummel MJ, et al. Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol. 2004. 22:658–663.
4. Chao Y, Yeh KH, Chang CJ, Chen LT, Chao TY, Wu MF, et al. Phase II study of weekly oxaliplatin and 24-h infusion of high-dose 5-fluorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer. 2004. 91:453–458.
5. Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999. 286:487–491.
6. Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res. 2008. 14:1291–1295.
7. Weaver DA, Crawford EL, Warner KA, Elkhairi F, Khuder SA, Willey JC. ABCC5, ERCC2, XPA and XRCC1 transcript abundance levels correlate with cisplatin chemoresistance in non-small cell lung cancer cell lines. Mol Cancer. 2005. 4:18.
8. Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995. 30:445–600.
9. Park DJ, Zhang W, Stoehlmacher J, Tsao-Wei D, Groshen S, Gil J, et al. ERCC1 gene polymorphism as a predictor for clinical outcome in advanced colorectal cancer patients treated with platinum-based chemotherapy. Clin Adv Hematol Oncol. 2003. 1:162–166.
10. Mort R, Mo L, McEwan C, Melton DW. Lack of involvement of nucleotide excision repair gene polymorphisms in colorectal cancer. Br J Cancer. 2003. 89:333–337.
11. Sreeja L, Syamala V, Hariharan S, Syamala VS, Raveendran PB, Sivanandan CD, et al. Glutathione S-transferase M1, T1 and P1 polymorphisms: susceptibility and outcome in lung cancer patients. J Exp Ther Oncol. 2008. 7:73–85.
12. Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007. 33:9–23.
13. Gately DP, Howell SB. Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer. 1993. 67:1171–1176.
14. Marsh S, McLeod HL. Cancer pharmacogenetics. Br J Cancer. 2004. 90:8–11.
15. Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehme A, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996. 56:4881–4886.
16. Dabholkar M, Vionnet J, Bostick-Bruton F, Yu JJ, Reed E. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J Clin Invest. 1994. 94:703–708.
17. Metzger R, Leichman CG, Danenberg KD, Danenberg PV, Lenz HJ, Hayashi K, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998. 16:309–316.
18. Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001. 19:4298–4304.
19. Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998. 19:275–280.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download