Abstract
Purpose
This study demonstrates that pharmacologic induction of heme oygenase-1 (HO-1) along with catalytic activation significantly modulated apoptosis of Jurkat cells induced by mycophenolic acid (MPA).
Methods
Cells were cultured with the presence or absence of MPA. Flow cytometric analysis was performed after propidium iodide staining. Western blotting of HO-1, Bcl, and Bax was also performed. Cells were stained 4'-6-Diamidino-2-phenylindole (DAPI) and measured by flow cytometry in the absence or presence of CoPPIX.
Results
Treatment of MPA decreased cell viability in a dose- and time-dependent manner. MPA-induced cell death was confirmed as apoptosis characterized by sub G0/G1 phase arrest. Expression of HO-1 assumes a pattern of decline after rising at the initial phase. CoPPIX, HO-1 inducer, significantly inhibited the cisplatin-induced apoptosis. Treatment of MPA resulted in reactive oxygen species (ROS) generation in Jurkat cells. CoPPIX attenuated ROS production in MPA-treated cells.
Figures and Tables
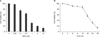 | Fig. 1MPA decreased the viability of Jurkat cells in a dose- and time-dependent manner. (A) Cells were treated with various concentrations of MPA for 24 hr and cell viability measured by MTT assay. (B) Cell viability was measured by MTT assay after 5µM MPA treatment for various times. *P<0.05 by student t-test, compared to control group. |
 | Fig. 2MPA increased the sub-G0/G1 fraction in Jurkat cells. (A) Cells were treated with 5µM MPA for various periods. After PI staining, sub-G0/G1 fraction (%) was measured by flow cytometry. (B) Apoptosis (%) in (A) was measured and plotted against time. Data represent the mean±S.D. of quadruplicates. *P<0.05 by Student's t-test, compared to control group. |
 | Fig. 3Production of H2O2 in MPA treated Jurkat cells. Cells were treated with 5µM MPA for various periods. Then, cells were incubated with the dye 2', 7'-dichlorofluorescin diacetate (5µM) and the fluorescence intensity of more than 10,000 cells was analyzed using flow cytometry. |
 | Fig. 4Effects of HO-1 expression on MPA treated-Jurkat cells in a time-dependent manner. Cells were treated with 5µM MPA for various periods. Cell lysates were separated on 15% SDS-PAGE and immunoblotted for anti-HO-1 and β-actin. The immunoreactive signals were visualized by ECL detection kit. |
 | Fig. 5Effect of CoPPIX on expression of HO-1 proteins in MPA treated Jurkat cells. Cells were treated with 5µM MPA in the absence and presence of 20µM MPA for 36 hr. Equal amounts of protein from cell lysate were subjected on 15% SDS-PAGE, transferred onto nitrocellulose membrane and immunoblotted with anti-HO-1 and anti-β-actin antibodies. The immunoreactive signals were visualized by ECL detection kit. |
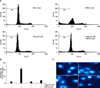 | Fig. 6Effect of CoPPIX, HO-1 inducer, in MPA induced apoptosis on Jurkat cells. (A) Cells were treated with 5µM MPA in the absence and presence of 20µM MPA for 48 hr. After PI staining, the fluorescence intensity of more than 10,000 cells was analyzed using a flow cytometry. (B) Apoptosis (%) in A was measured and plotted against dose. Data represent the mean±standard deviation (S.D.) of quadruplicates. (C) Cells were stained with 10µg/ml DAPI and visualized under fluorescence microscope. |
 | Fig. 7Effects of CoPPIX on expression of Bcl-2 and Bax proteins in MPA treated Jurkat cells. Cells were treated with MPA for 36 hr in the absence or presence of CoPPIX. Equal amounts of protein from cell lysate were subjected on 15% SDS-PAGE, transferred onto nitrocellulose membrane and immunoblotted with anti-Bcl-2, anti-Bax and anti-β-actin antibodies. The immunoreactive signals were visualized by ECL detection kit. |
References
1. Florey HW, Jennings MA, Gilliver K, Sanders AG. Mycophenolic acid: an antibiotic from penicillium brevicompactum Dierckx. Lancet. 1946. 247:46–49.
2. Allison AC, Kowalski WJ, Muller CD, Eugui EM. Mechanisms of action of mycophenolic acid. Ann N Y Acad Sci. 1993. 696:63–87.
3. Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000. 47:85–118.
4. Natsumeda Y, Carr SF. Human type I and II IMP dehydrogenases as drug targets. Ann N Y Acad Sci. 1993. 696:88–93.
5. Konno Y, Natsumeda Y, Nagai M, Yamaji Y, Ohno S, Suzuki K, et al. Expression of human IMP dehydrogenase types I and II in Escherichia coli and distribution in human normal lymphocytes and leukemic cell lines. J Biol Chem. 1991. 266:506–509.
6. Nagai M, Natsumeda Y, Konno Y, Hoffman R, Irino S, Weber G. Selective up-regulation of type II inosine 5'-monophosphate dehydrogenase messenger RNA expression in human leukemias. Cancer Res. 1991. 51:3886–3890.
7. Allison AC, Eugui EM. The design and development of an immunosuppressive drug, mycophenolate mofetil. Springer Semin Immunopathol. 1993. 14:353–380.
8. Goda N, Suzuki K, Naito M, Takeoka S, Tsuchida E, Ishimura Y, et al. Distribution of heme oxygenase isoforms in rat liver. Topographic basis for carbon monoxide-mediated microvascular relaxation. J Clin Invest. 1998. 101:604–612.
9. Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, Tamatani T, et al. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res. 1999. 85:663–671.
10. Gao Z, Huang K, Xu H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol Res. 2001. 43:173–178.
11. Lee WA, Gu L, Miksztal AR, Chu N, Leung K, Nelson PH. Bioavailability improvement of mycophenolic acid through amino ester derivatization. Pharm Res. 1990. 7:161–166.
12. Sollinger HW. U.S. Renal Transplant Mycophenolate Mofetil Study Group. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. Transplantation. 1995. 60:225–232.
13. Lemos FB, Ijzermans JN, Zondervan PE, Peeters AM, van den Engel S, Mol WM, et al. Differential expression of heme oxygenase-1 and vascular endothelial growth factor in cadaveric and living donor kidneys after ischemia-reperfusion. J Am Soc Nephrol. 2003. 14:3278–3287.
14. Park SH, Jang JH, Li MH, Na HK, Cha YN, Surh YJ. Nrf2-mediated heme oxygenase-1 induction confers adaptive survival response to tetrahydropapaveroline-induced oxidative PC12 cell death. Antioxid Redox Signal. 2007. 9:2075–2086.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download