Abstract
Purpose
The aim of this study was to compare the short and long-term outcomes following carotid endarterectomy (CEA) with either primary closure (PC) or patch angioplasty (PAT) performed by single center vascular surgeons.
Methods
Between November 1994 and March 2008, a total of 366 patients underwent 401 consecutive primary CEA procedures at our institution. We retrospectively reviewed patients' medical records. Two vascular surgeons prefer routine PC and one vascular surgeon prefer routine patch closure using bovine pericardial patch. Postoperative neurologic complications were determined by clinical neurologists. Restenosis was defined as >50% stenosis on follow-up duplex scan. Data was analyzed to compare the early (≤30 days) and late results of CEA between PC group and PAT group.
Results
The mean follow-up duration was significantly longer in the PC group than that in the PAT group (61.7 months vs. 41.2 months, P<0.001). Coronary artery disease and combined CEA with coronary artery bypass were more common in the PAT group (39% vs. 55%, P<0.002; 4% vs. 12%, P<0.004). Perioperative ipsilateral TIA/stroke rates in the PC and PAT groups were 1.5% and 0.7% (PC=4/270 vs. PAT=1/131, P=0.564). Regarding late outcomes, Kaplan-Meier analysis failed to show any difference between 2 groups on freedom from ipsilateral transient ischemic attack (TIA)/stroke, freedom from restenosis and TIA/stroke-free survival (P=0.851, P=0.232, P=0.103, log-rank test).
Carotid endarterectomy (CEA) is the gold standard to reduce stroke. However, there is controversy among the method of arterial wall closure following CEA. Previous clinical studies have shown closure with patch angioplasty (PAT) reduces the perioperative stoke rate and the restenosis rate.(1-6) Other authors, however, have reported PAT is not superior to primary closure (PC).(7-9)
Carotid lesions in Asians have unique characteristics when compared with those in the West. The atherosclerotic plaque tends to involve the more distal internal carotid artery (ICA).(10) For this reason, PAT would seemingly be superior to PC, especially in Asians. However, few studies in Asian patients report the outcome after PAT vs. PC.
In the earlier paper, we reported equivalent results of PC when compared with others' results of PAT.(11) We noted also that in the previous randomized studies comparing PAT and PC, the surgeon experience was rarely considered. CEAs might be performed by not only experienced surgeons, but also by trainees. We hypothesized that the surgeon experience may influence the outcome of CEA with PC. In this study, we aimed to compare the perioperative and long-term outcomes between PC and PAT performed by experienced surgeons at our institution.
Between November 1994 and March 2008, a total of 366 patients underwent 401 consecutive primary CEA operations at Samsung Medical Center. We retrospectively reviewed the patients' medical records. The indications for CEA were previously described by us,(11) and summarized here.(1) symptomatic stenosis of >70%,(2) symptomatic stenosis of 50~69% with type C plaque ulcer (more than 40 mm2 in plaque ulcer),(3) asymptomatic stenosis of >70% with contralateral ICA occlusion, and(4) asymptomatic stenosis of >70% with type C plaque ulceration. Degree of stenosis was calculated according to the method of NASCET (North American Symptomatic Carotid Endarterectomy Trial). All CEAs were performed by three senior vascular surgeons under general anesthesia and routine shunting using a Pruitt-Inahara carotid shunt (Number 2004-49, LeMaitre Vascular, St. Petersburg, FL, USA). Two vascular surgeons prefer routine PC and one vascular surgeon prefer routine patch closure using bovine pericardial patch.
All the patients underwent postoperative evaluation by both vascular surgeons and neurologists. Duplex ultrasound scanning was performed postoperatively at 1 month, 3 months and 6 months, then every 6 months thereafter. Postoperative neurologic complications such as transient ischemic attack (TIA), stroke and cranial nerve palsy were determined clinically by the neurologist. Restenosis was defined as ≥50% stenosis on a duplex scan; ICA peak systolic velocity ≥125 cm/s and diameter reduction ≥50%. Aspirin (100 mg/day) was routinely administered life-long after surgery.
All CEA patients were categorized to either the PC group or the PAT group. Demographic and clinical data of the groups were compared, including comorbidities, previous history of TIA or stroke, and contralateral ICA occlusion. Data was analyzed to compare early (≤30 days) and late results following CEA in the two groups. Categorical variables were compared using chi-square tests or Fisher's exact test, and the continuous variables were examined using Student t-test. Freedom from ipsilateral TIA/stroke, freedom from any TIA/stroke, freedom from restenosis and TIA/stroke-free survival were calculated by Kaplan-Meier method and compared by log-rank test. P-values<0.05 were considered to be statistically significant.
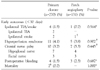
Table 1 shows the demographic and clinical data of the 2 groups. The mean follow-up duration was longer in the PC group (61.7 vs. 41.2 months, respectively, P<0.001). Coronary artery disease (39% vs. 55%, respectively, P=0.002) and combined CEA+coronary artery bypass surgery (4% vs. 12%, respectively, P=0.004) was more common in the PAT group. There were no other significant differences.
Table 2 shows early and late outcomes following CEA according to closure type. Perioperative (<30 days) ipsilateral TIA/stroke rate in the PC and PAT groups were noted to be 1.5% and 0.7%, respectively (PC=4/270 vs. PAT=1/131, P=0.564). There was no significant difference between the two groups in postoperative incidence of hyperperfusion syndrome, cranial nerve palsy, myocardial infarction and postoperative bleeding. One perioperative stroke due to thrombosis occurred in the PAT group. Emergency angiography revealed ICA thrombosis, and catheter-directed thrombectomy and thrombolytic therapy was performed. This revealed underlying ICA stenosis which was treated with a carotid stent. In the PC group, there were two perioperative deaths caused by vertebral artery thrombosis in one and pneumonia followed by respiratory failure in a second patient.
During the late follow-up, ipsilateral TIA/stroke was detected in 2 patients (0.7%) who underwent PC; there was no TIA/stroke in the PAT group. Freedom from ipsilateral TIA/stroke and freedom from any TIA/stroke were not different between 2 groups (Fig. 1). Six (2.2%) and three (2.3%) restenoses occurred in the PC and PAT groups, respectively. Fig. 2 demonstrates Kaplan-Meier analysis of restenosis-free rate and TIA/stroke-free survival rate. Five-year, 10-year restenosis-free rates were 98%, 97% in PC group and 95%, 95% in PAT group (P=0.232, log-rank test). For the treatment of restenosis, one redo CEA and two carotid stent procedures were performed for the 3 patients of the PC group. All other patients are undergoing regular follow-up without secondary intervention. Five-year, 10-year TIA/stroke-free survival rates were slightly higher in PAT group, however, they were not statistically significant (87%, 69% vs. 78%, 58%, P=0.103, log-rank test).
Recently, the European Society for Vascular Surgery (ESVS) guidelines of invasive treatment for carotid stenosis suggested that PAT is preferable to PC.(12)
The rationale for performing PAT is that it increases the diameter of the arterial and this can reduce the effect of intimal hyperplasia, which can cause restenosis.(13) Further, a wider lumen serves the superior flow characteristics of the internal carotid artery in terms of not generating early thrombosis and hyperplasia.(14)
The clinical outcomes of the previously published randomized trials comparing arteriotomy closure are illustrated in Table 3. In some trials, the perioperative stroke rate and restenosis rate was significantly lower following PAT compared to PC.(1-6) AbuRahma et al.(15) compared the outcomes following bilateral CEAs in the patients who underwent PC on one side and PAT on the contralateral side. They reported that PAT showed superior result compared to PC for the same systemic condition. However, in other trials, the results of PAT were not superior to those of PC.(7-9) Therefore, some authors have suggested conducting a large multicenter randomized controlled trial in order to obtain reliable evidence on the risks and benefits of PAT compared to PC.(16)
In terms of selective PC, some authors have reported that PAT of a larger carotid artery is unnecessary(17) and others showed that PC is safe and durable when the arteriotomy and endarterectomy end points are within the carotid bulb.(18) Byrne et al.(19) suggested that PC can be safely practiced in large-caliber ICAs (>6 mm). Still, no randomized control trial has yet been performed.
We have previously reported excellent outcomes from our institution following CEA with PC, when compared with others' results of CEA with PAT.(11) We assumed that postoperative restenosis is related to the remnant synthetic type of vascular smooth muscle cells (VSMCs) rather than the type of closure (e.g., PC and PAT), on the basis of microscopic examination of the endarterectomized ICA wall. Although our results after PC were satisfactory in the previous study, we wanted to know if PAT could result in a potentially superior outcome at our institution. Given a lack of significant difference in both the early and late complications between the groups, there appears to be no benefit to PAT at our institution.
In previous randomized studies, the surgeon experience was not considered. Vascular surgery is highly dependent on the surgeon's skill and experience. Pearce et al.(20) reported a doubling of surgeon volume was associated with a 4% reduction in the risk for an adverse outcome following CEA. Cowan et al.(21) also showed that the mortality rate and the perioperative stroke rate were significantly lower in the CEAs performed by high-volume surgeons (≥30 procedures/year).
In this study, all the CEAs were performed by experienced vascular surgeons rather than by surgeons in training. Based on our results, we assume that closure type does not correlate with postoperative stroke or restenosis rates. PAT can, however, reduce the effect of technical errors. PAT is currently more popular than PC, so surgeons generally have more experience with this technique when in training. This inexperience with PC in concern over restenosis and ultimately has led to a preference for PAT restenosis despite the potential disadvantages of PAT such as the increased the clamp and shunt time, the risk of patch rupture, pseudoaneurysm formation, patch infection and thromboembolism from aneurismal carotid dilatation.(8,22,23)
In summary, our results suggest that for experienced surgeons, PC following CEA is not necessarily inferior to PAT. PC is a safe and durable procedure and routine patching is not necessary. This study has important limitations, however, mostly stemming from retrospective design and its relatively small sample size for statistical analysis. Another is a discrepancy of follow-up duration between two groups. In the future, a prospective randomized study is warranted and surgeon experience should be considered in such a study.
Figures and Tables
 | Fig. 1Kaplan-Meier curves comparing freedom from ipsilateral TIA/stroke (A) and freedom from any TIA/stroke (B). |
Table 3
Clinical outcomes of the randomized trials and our previous study regarding the types of arteriotomy closure

*TIA = transient ischemic attack; †PC = primary closure; ‡PAT = patch angioplasty; §43 saphenous vein patches and 47 polytetrafluoroethylene (PTFE) patches; ∥70 saphenous vein patches, 60 jugular vein patches and 134 PTFE patches; ¶Bilateral carotid endarterectomies with primary closure on one side and patch angioplasty on the other side.
ACKNOWLEDGEMENTS
We thank Dr. BB Lee, one of the senior surgeons in this study. Now, he is a professor of Surgery & Director, Center for Vein, Lymphatics and Vascular Malformation, Georgetown University School of Medicine, Washington, DC, USA.
References
1. Ranaboldo CJ, Barros D'Sa AA, Bell PR, Chant AD, Perry PM. The Joint Vascular Research Group. Randomized controlled trial of patch angioplasty for carotid endarterectomy. Br J Surg. 1993. 80:1528–1530.
2. AbuRahma AF, Khan JH, Robinson PA, Saiedy S, Short YS, Boland JP, et al. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: perioperative (30-day) results. J Vasc Surg. 1996. 24:998–1006.
3. AbuRahma AF, Robinson PA, Saiedy S, Kahn JH, Boland JP. Prospective randomized trial of carotid endarterectomy with primary closure and patch angioplasty with saphenous vein, jugular vein, and polytetrafluoroethylene: long-term follow-up. J Vasc Surg. 1998. 27:222–232.
4. Eikelboom BC, Ackerstaff RG, Hoeneveld H, Ludwig JW, Teeuwen C, Vermeulen FE, et al. Benefits of carotid patching: a randomized study. J Vasc Surg. 1988. 7:240–247.
5. De Letter JA, Moll FL, Welten RJ, Eikelboom BC, Ackerstaff RG, Vermeulen FE, et al. Benefits of carotid patching: a prospective randomized study with long-term follow-up. Ann Vasc Surg. 1994. 8:54–58.
6. Lord RS, Raj TB, Stary DL, Nash PA, Graham AR, Goh KH. Comparison of saphenous vein patch, polytetrafluoroethylene patch, and direct arteriotomy closure after carotid endarterectomy. Part I. Perioperative results. J Vasc Surg. 1989. 9:521–529.
7. Katz D, Snyder SO, Gandhi RH, Wheeler JR, Gregory RT, Gayle RG, et al. Long-term follow-up for recurrent stenosis: a prospective randomized study of expanded polytetrafluoroethylene patch angioplasty versus primary closure after carotid endarterectomy. J Vasc Surg. 1994. 19:198–203.
8. Clagett GP, Patterson CB, Fisher DF Jr, Fry RE, Eidt JF, Humble TH, et al. Vein patch versus primary closure for carotid endarterectomy. A randomized prospective study in a selected group of patients. J Vasc Surg. 1989. 9:213–223.
9. Myers SI, Valentine RJ, Chervu A, Bowers BL, Clagett GP. Saphenous vein patch versus primary closure for carotid endarterectomy: long-term assessment of a randomized prospective study. J Vasc Surg. 1994. 19:15–22.
10. Kim GE, Kwon TW, Cho YP, Kim HS. Carotid endarterectomy prospective study. J Korean Surg Soc. 1998. 55:265–273.
11. Kim DI, Moon JY, Lee CH, Kim DY, Jang YS, Kim GM, et al. Primary closure after a carotid endarterectomy. Surg Today. 2007. 37:187–191.
12. Liapis CD, Bell PR, Mikhailidis D, Sivenius J, Nicolaides A, Fernandes e Fernandes J, et al. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg. 2009. 37:1–19.
13. Deriu GP, Ballotta E, Bonavina L, Grego F, Alvino S, Franceschi L, et al. The rationale for patch-graft angioplasty after carotid endarterectomy: early and long-term follow-up. Stroke. 1984. 15:972–979.
14. Archie JP Jr. Prevention of early restenosis and thrombosisocclusion after carotid endarterectomy by saphenous vein patch angioplasty. Stroke. 1986. 17:901–905.
15. AbuRahma AF, Robinson PA, Saiedy S, Richmond BK, Khan J. Prospective randomized trial of bilateral carotid endarterectomies: primary closure versus patching. Stroke. 1999. 30:1185–1189.
16. Counsell C, Salinas R, Warlow C, Naylor R. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database Syst Rev. 2000. CD000160.
17. Golledge J, Cuming R, Davies AH, Greenhalgh RM. Outcome of selective patching following carotid endarterectomy. Eur J Vasc Endovasc Surg. 1996. 11:458–463.
18. Archie JP Jr. A fifteen-year experience with carotid endarterectomy after a formal operative protocol requiring highly frequent patch angioplasty. J Vasc Surg. 2000. 31:724–735.
19. Byrne J, Feustel P, Darling RC 3rd. Primary closure, routine patching, and eversion endarterectomy: what is the current state of the literature supporting use of these techniques? Semin Vasc Surg. 2007. 20:226–235.
20. Pearce WH, Parker MA, Feinglass J, Ujiki M, Manheim LM. The importance of surgeon volume and training in outcomes for vascular surgical procedures. J Vasc Surg. 1999. 29:768–776.
21. Cowan JA Jr, Dimick JB, Thompson BG, Stanley JC, Upchurch GR Jr. Surgeon volume as an indicator of outcomes after carotid endarterectomy: an effect independent of specialty practice and hospital volume. J Am Coll Surg. 2002. 195:814–821.
22. O'Hara PJ, Hertzer NR, Krajewski LP, Beven EG. Saphenous vein patch rupture after carotid endarterectomy. J Vasc Surg. 1992. 15:504–509.
23. Borazjani BH, Wilson SE, Fujitani RM, Gordon I, Mueller M, Williams RA. Postoperative complications of carotid patching: pseudoaneurysm and infection. Ann Vasc Surg. 2003. 17:156–161.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download