INTRODUCTION
Littoral cell angioma (LCA) of the spleen was first documented in a case described by Falk et al.(1) in 1991. LCA is distinctive in that they show both epithelial and histiocytic properties of the normal splenic littoral cells lining the sinus channels of the splenic red pulp.(1) Most LCA are detected in adults, but have been reported in children.(1) The clinical symptoms and signs of LCA may include abdominal pain, splenomegaly, thrombocytopenia, anemia, splenic rupture with hemoperitoneum, weakness or fatigue.(2,3) Other case of LCA may be completely asymptomatic. In this report, we present a case of littoral cell angiomatosis associated with liver cirrhosis.
CASE REPORT
The patient was a 34-year-old woman who visited for further evaluation of her liver cirrhosis. She was diagnosed as chronic viral hepatitis C, two years ago. Five months ago, she developed manifestation of cirrhosis. She drank 7~14 bottles of distilled liquor/week for the last six years. Her surgical history was unremarkable.
She showed symptom of hepatic encephalopathy including drowsy mentality. The laboratory abnormality included elevated liver enzymes and total bilirubin level (ALP=69 IU/L, AST=80 IU/L, total bilirubin=14.5 mg/dl). Prothrombin time (PT, 21.5 sec) and activated partial thromboplastin time (aPTT, 39.7 sec) were prolonged. The viral markers were as follows: HBs Ag/Ab (-/-), Anti HCV Ab (+), HCV RNA (7×10 IU/ml).
Abdominal computed tomography (CT) showed multiple benign looking nodules in both hepatic lobes. No definite splenic abnormality is identified by CT scan and sonography.
She has been diagnosed alcohol and hepatitis C associated liver cirrhosis. Liver transplantation was performed due to cirrhosis. Splenectomy was performed due to splenomegaly complication such as anemia (Hb, 8.0 g/dl) and thrombocytopenia (Platelet, 99,000/mm3). Explantated liver showed diffusely cirrhotic nodules (up to 1.0 cm in greatest dimension). The splenomegaly (375 g) is identified, grossly. The pathologic diagnosis of explantated liver was mixed macro and micronodular cirrhosis. Also microscopically, multiple anastomosing vascular channels, with pseudopapillary projection and cyst-like space (up to 0.3 cm in greatest dimension) were noted in splenic parenchyma. The lining cells showed bland cytologic characteristics including vesicular nuclei and pale, eosinophilic cytoplasm (Fig. 1). No mitosis or cytological atypia was identified.
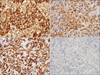
On immunohistochemical examination, the lining cell was positive for CD21, CD31, CD68, CD163 and negative for CD8, CD23, CD4 and CD34 (Fig. 2). The ki-67 labeling index was less than 1%. These histomorphologic and immunohistochemical findings were consistent with littoral cell angioma. The patient was discharged from the hospital on the 74th day.
DISCUSSION
Littoral cell angioma is a benign lesion, believed to represent proliferation or the sinus endothelial of the splenic red pulp. LCA of the spleen may arise at any age (1~77 years; median age, 50 years), with no sex-based preponderance (female : male ratio, 5 : 3).(4,5)
Etiology is unknown due to limited number of cases. It has been proposed that the chronic infection, autoimmune disorders, inborn metabolic diseases, immune system dysfunction and other malignancies play a role in development of LCA. Autoimmune disorders such as inflammatory bowel disease and ankylosing sponylitis have been associated with LCA. Immunosuppression by steroids and cyclosporin has been suggested that they were associated with development of LCA. Harmon et al.(6) reported half of the 74 cases, they described were associated with some immune dysregulation including malignancy, drug use, or disease. Other malignancies, associated with LCA include: hematologic (lymphoma), colorectal, pulmonary, ovarian, pancreatic, renal, cutaneous (melanoma), mammary, testicular and thyroid carcinoma.(6) However, another malignancy was not identified by colonoscopy, gastroduodenoscopy and CT scan at 3 month follow up period. And autoimmune disorders such as inflammatory bowel disease and ankylosing sponylitis were not identified by colonoscopy and physical exam after follow up.
Five cases, associated with viral hepatitis were reported (3 hepatitis B, 1 Hepatitis C, 1 Hepatitis A virus).(6,7) Kim et al.(8) reported a case of LCA in a patient with hepatitis B-associated cirrhosis. Our case is second case of LCA, that is associated cirrhosis. And viral hepatitis C associated LCA was not reported in Korea.
Macroscopically, LCA is characterized by single or multiple spongy red-brown nodules that are blood-filled. The size of these lesions may range from 0.1 cm to 11 cm in diameter. Usually, the spleen may be enlarged or normal size.
Histologically, LCA is characterized by anastomosing vascular channels with variable-sized cavernous spaces replacing the red pulp. The channels have irregular lumina featuring papillary projections and cystic spaces. The luminal endothelial cells frequently detach into vascular spaces. The cells show no cytologic atypia and little mitosis. If any nuclear atypia or high mitosis is identified, this finding suggests malignancy such as littoral cell angiosarcoma or littoral cell hemangioendothelioma. By immunohistochemical staining, the tumor expresss immunoreactivity with endothelial markers (factor VIII, CD31) and histiocytic markers (CD68, lysozyme, CD21), which reveal the tumor's dual histiocytic/endothelial differentiation. Although sinus lining cells and some cases of littoral cell angioma have been reported to show CD8 expression, Rosso et al. mentioned negativity of CD8 immunostaining was seen in most case of LCA.(9) In our case, the microscopic findings and immunohistochemical profiles were typical features of a LCA.
LCA tends to be a benign tumor but may behave unpredictably. Laparoscopic or laparotomic splenectomy is the definitive treatment. As it can coexist with a variety of malignancies, strict work up for other malignancy and a long term follow-up are required.
In summary, we describe an incidental case of LCA occurring with liver cirrhosis, hepatitis C virus associated. Although the great majority of LCA is benign disease, it can be associated with other malignancies and immune system disorders. Therefore, clinically associated condition must be screened in a patient with apparently isolated LCA.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download