Abstract
Purpose
The five-year survival rates of patients with stage III colorectal cancer have been reported widely ranging from 22 to 69 percent. Hence, reliable substaging is important for the management of stage III colorectal cancer patients. Therefore, we tried to assess the substages and investigate the possibility of other discriminating numbers for nodal substaging.
Methods
The 381 patients with node-positive colorectal cancer who had undergone surgery, were retrospectively categorized by the number of positive nodes. The patients were grouped in five ways, and each grouping was divided into two subgroups according to the number of positive nodes. The subgroups of each grouping were as follows; in LN1 group, N1=1, N2>1; in LN2 group, N1=2, N2>2; in LN3 group, N1=3, N2>3; in LN4 group, N1=4, N2>4; in LN5 group, N1=5, N2>5. We compared the survival rate of each groups.
Results
Node-positive patients had a five-year survival rate of 55.2 percent. The statistical differences between the N1 and N2 subgroups of each grouping were as follows: LN1 group (P=0.0128), LN2 group (P=0.0052), LN3 group (P=0.6268), LN4 group (P=0.1480), and LN5 group (P=0.6875).
Conclusion
There were significant differences in the five-year survival rates between N1 and N2 in the LN1 group and LN2 group, but there were no differences between N1 and N2 in the other groupings. These data raise the possibility that a novel N1~N2 substaging (N1: 1~2; N2: >2) is superior to the current N1~N2 substaging (N1: 1~3; N2: >3).
In patients with colorectal cancer, the most important indicator of prognosis, next to the presence of distant metastasis, is the presence of lymph node metastasis.(1) Therefore, the designation of stage III colorectal cancer has been based on nodal involvement. However, the five-year survival rates of patients with stage III colorectal cancer have been reported widely ranging from 22 to 69 percent.(2,3) Accordingly, reliable lymph node substaging is important for the management of stage III colorectal cancer patients.
The Gastrointestinal Tumor Study Group (GITSG),(4) the National Surgical Adjuvant Breast/Bowel Project,(5) and the Japanese Research Society for Cancer of the Colon and Rectum(6) subdivided colorectal cancers with positive lymph nodes into one to four and five or greater positive nodes. While the American Joint Committee on Cancer and the Union Internationale Contre le Cancer (AJCC/UICC) subdivided colorectal cancers into one to three and four or more positive nodes in 2002,(7) many methods have been proposed for the differentiation among patients with node-positive colorectal cancer. However, there still is controversy concerning the influences of these classifications. Therefore, we assessed the lymph node substages and investigated the possibility of other discriminating numbers for nodal substaging.
The subjects of this retrospective study were 381 patients with stage III colorectal cancer who were chosen from 858 patients who had surgery for colorectal cancer between March 1991 and November 2004.
Based on the number of positive lymph nodes, the patients were grouped by five ways for five different observations. The first grouping, LN1 group, was divided into two subgroups; N1 with one positive lymph node and N2 with more than one. The second grouping, LN2 group, was divided into N1 with two positive lymph nodes and N2 with more than two. In LN2 group, patients with one positive lymph node were excluded. In the same way, LN3 group was divided into N1 with three positive lymph nodes and N2 with more than three, not including patients with less than three positive lymph nodes. LN4 group was divided into N1 with four positive lymph nodes and N2 with more than four, excluding patients with less than four positive lymph nodes. LN5 group was divided into N1 with five positive lymph nodes and N2 with more than five, not including patients with less than five positive lymph nodes. For each grouping, the survival rates of N1 and N2 were compared.
In an inital study, we had grouped the patients by a different method, not excluding any patients. For example, LN3 group had been divided into N1 with three positive lymph nodes and less and N2 with more than three. However, we found that there were statistical differences in survival rates between N1 and N2 in this grouping method. The main reason for these differences was that the survival rate of the N1 subgroup included all cases which showed far better prognosis. Therefore, we decided not to include patients with less numbers of positive lymph nodes in each grouping as noted above. We also decided to use the Kaplan-Meier analysis.
All operations were performed by one staff of the two colorectal surgeons. Curative resection included the main lymphovascular supply to the bowel. For proximal colon cancers, lymphadenectomy was extended to the origin of the ileocolic, right colic, and middle colic arteries. For distal colon cancers and rectal cancers, lymphadenectomy was extended to the origin of the inferior mesenteric artery. Total mesorectal excision was performed in all patients with cancers of the middle and lower rectum. At least 2 cm of normal mucosa from the lower edge of the tumors were resected.
The same team of pathologists examined all the surgical specimens of lymph nodes, and the same technique for lymph node assessment was utilized during the period of the study. Lymph nodes were identified by palpation, and routine histological examination was performed with hematoxylin and eosin stain. No special clearance or staining techniques were employed.
All patients with stage III colon cancer received adjuvant chemotherapy with a drug regimen of 425 mg/m2/day 5-fluorouracil and 20 mg/m2/day leucovorin, 5 days/week, every 4 weeks, for 6 months. Patients with T4N1M0 or T4N2M0 rectal cancer received 5,040 cGy irradiation therapy after surgery, divided into 28 daily doses of 180 cGY each (5 doses/week for 5 weeks and 3 days).
The survival curves of the N1 and N2 subgroups in all the groupings were calculated by the Kaplan-Meier method and compared for statistical significance using the Log-rank test. The statistical significance between tumor location and the number of lymph nodes was analyzed using ANOVA. The significance level was set at P=0.05 for each analysis, and all calculations were performed by SPSS software (ver. 11, SPSS Inc., Chicago, IL, USA).
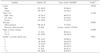
The overall five-year survival rate including stage IV was 64.5%±0.3 (standard error of the mean) and the mean follow up period was 49.7 months for patients with colorectal cancer. The distribution of the tumors as stage was stage I 19.9% (n=171), stage II 29.9% (n=257), stage III 44.4% (n=381), and stage IV 5.8% (n=49). Among these patients, clinical data of 381 patients who underwent curative surgery for stage III colorectal cancer were reviewed retrospectively. There were 222 (58.3%) male and 159 (41.7%) female patients with a mean age of 58.4 (range 19~80) years and a five-year survival rate was 55.2%±0.4 (standard error of the mean)(Table 1).
The five year survival rates of each nodal groups were as follows (number of cases): 1 positive node, 65.90% (102); 2 positive nodes, 64.41% (64); 3 positive nodes, 40.30% (39); 4 positive nodes, 45.16% (36); 5 positive nodes, 40.14% (31); >5 positive nodes, 40.82% (109) (Table 1). The statistical differences of five-year survival rates between N1 and N2 subgroups were as follows: LN1 group (N1: 1; N2: >1; P=0.0128), LN2 group (N1: 2; N2: >2; P=0.0052), LN3 group (N1: 3; N2: >3; P=0.6268), LN4 group (N1: 4; N2: >4; P=0.1480), and LN5 group (N1: 5; N2>5; P=0.6875). There were significant differences in five-year survival rates between N1 and N2 in the LN1 group and LN2 group, but there was no difference between N1 and N2 in the other groups (Fig. 1).
Age and histological type had additional influence upon survival. The numbers of patients with T1, T4 were 3 (0.9%), 5 (1.2%) respectively and these numbers were too small to achieve statistical significance of survival according to each T-substage in stage III colorectal cancers. Thus, we divided the T-substage into T1~2 and T3~4 and obtained statistical difference in five-year survival rates (P=0.0368). Although the patients with left colon cancer who underwent curative surgery had better survival rates than the patients with colon cancers in other sites, the statistical differences of survival according to the location of the colon cancers were not determined (P=0.1104). Among 381 stage III patients, 54 (14.1%) patients had cancer in the right colon, 95 (25.0%) patients in the left colon, and 232 (60.9%) patients in the rectum (Table 1).
The average number of nodes examined in each patient was 18.3 (range 8~64) and the average number of positive lymph nodes in each patient was 6.0 (range 1~63). The number of lymph nodes examined in tumors of the right colon was greater than the others, and there was statistical significance between tumor location and the number of harvested lymph nodes. The numbers of lymph nodes examined corresponding to tumor location were as follows (mean±standard deviation); right colon: 22.8±13.8; left colon: 16.5±7.0; rectum: 17.4±9.2 (P=0.002).
Multiple prognostic factors affect the survival of patients with primary colorectal cancer. The presence of distant metastasis is the most important, and the second indicator is the presence of lymph node metastasis.(1) Additional pathologic variables include the extent of primary tumor, mural penetration, primary site in the colon, degree of differentiation, presence of a mucinous histology, tumor configuration, and lymphatic vessel invasion.(1) Therefore, substaging of regional lymph node metastasis is very important for the management of colorectal cancer patients. However, the five year survival rates of stage III colorectal cancer patients have been reported widely ranging from 22% to 69%.(2,3)
For colorectal cancer, staging strategies recommended by Dukes and modified by Kirklin et al.(8) and Astler and Coller(9) were based on careful analyses of mural involvement and the presence of regional nodal involvement, although the number of regional nodes involved was not considered. In the year 1935, Dukes and his colleagues proposed that Class C tumors be further subdivided into C1 and C2 subsets, the latter indicative of nodal involvement that had reached the glands at the uppermost point of ligature of the mesenteric blood vessels and the former describing glandular spread below that level.(10) In the year 1958, Dukes correlated increasing numbers of involved lymph nodes, in subgroups of 1, 2~5, 6~10, and >10 involved nodes.(11) The Gastrointestinal Tumor Study Group (GITSG),(4) the National Surgical Adjuvant Breast/Bowel Project,(5) and the Japanese Research Society for Cancer of the Colon and Rectum(6) substaged colorectal cancers with the number of positive lymph nodes (C1: 1~4; C2: >4). Additionally the American Joint Committee on Cancer and the Union Internationale Contre le Cancer (AJCC/UICC) subdivided colorectal cancers according to the number of positive lymph nodes in 2002 (N1: 1~3; N2: >3).(7) Thus, various methods have been proposed for the differentiation among patients with node-positive colorectal cancer.
Sternberg et al.(12) reported that the TNM N1/N2 method (N1: 1~3; N2: >3) failed to separate the 171 node-positive cohort into subsets that differed significantly with respect to either cancer recurrence or cancer-related death. The GITSG C1/C2 subsets (N1: 1~4; N2: >4) differed significantly only in cancer-related survival rates but not in disease free survival rates. Furthermore, our study demonstrated that there was no difference in five-year survival rates between N1 and N2 in the LN3 group (N1: 3; N2: >3) and LN4 group (N1: 4; N2: >4), but there were significant differences of five-year survival rates between N1 and N2 in the LN1 group (N1: 1; N2: >1) and LN2 group (N1: 2; N2: >2). These data suggest the possibility that a novel N1~N2 substaging (N1: 1~2; N2: >2) may be superior to the current N1~N2 substaging (N1: 1~3; N2: >3).
Since the stage III group is defined by the identification and quantification of mesenteric nodes, accuracy of staging is directly proportional to the aggressiveness of surgical resection and nodal identification in patients with colorectal cancer. Low lymph node harvests will lead to a reduction in the number of stage III cases. This observation may have several important implications for patients, as they may be denied life saving adjuvant chemotherapy. The AJCC(13) and the College of American Pathologists(14) have recommended examination of at least 12 lymph nodes to assume identification of stage III patients. However, a French population based study reported that there was no significant risk of misclassification above a number of eight retrieved lymph nodes.(15) While a recent comprehensive study of lymph node harvests found that there was no safe minimum number that could guarantee identification of node involvement, it recommended that all lymph nodes in the lymphatic field of a colorectal cancer should be removed for histopathological assessment.(16) We believe that the numbers of harvested lymph nodes (mean: 18.3; range: 8~64) in this study were enough to assess the lymph node substages.
In our study, there were statistical differences in five-year survival rates between N1 and N2 in the LN1 group and LN2 group, but there were no differences between N1 and N2 in the other groupings. These data raise the possibility that a novel N1~N2 substaging (N1: 1~2; N2: >2) is superior to the current N1~N2 substaging (N1: 1~3; N2: >3).
With further clinical data, there is a possibility that our survival analysis by this method will result in the novel N1~N2 substaging of the TNM stage, and may have practical therapeutic implications in the future.
Figures and Tables
Fig. 1
Kaplan-Meier estimates of the survival rates overall and by 5 groupings; LN1 group (N1: 1; N2: >1), LN2 group (N1: 2; N2: >2), LN3 group (N1: 3; N2: >3), LN4 group (N1: 4; N2: >4), and LN5 group (N1: 5; N2>5). The statistical differences between N1 and N2 categories of each group were compared for statistical significance using the log-rank test: LN1 group (P=0.0128), LN2 group (P=0.0052), LN3 group (P=0.6268), LN4 group (P=0.1480), and LN5 group (P=0.6875) (N1: □-□, N2: +-+).

References
1. Cohen AM, Tremiterra S, Candela F, Thaler HT, Sigurdson ER. Prognosis of node-positive colon cancer. Cancer. 1991. 67:1859–1861.
2. Enker WE, Laffer UT, Block GE. Enhanced survival of patients with colon and rectal cancer is based upon wide anatomic resection. Ann Surg. 1979. 190:350–360.
3. Tang R, Wang JY, Chen JS, Chang-Chien CR, Tang S, Lin SE, et al. Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J Am Coll Surg. 1995. 180:705–712.
4. Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985. 312:1465–1472.
5. Wolmark N, Fisher B, Wieand HS. The prognostic value of the modifications of the Dukes' C class of colorectal cancer. An analysis of the NSABP clinical trials. Ann Surg. 1986. 203:115–122.
6. Japanese Research Society for Cancer of the Colon and Rectum. General rules for clinical and pathological studies on cancer of the colon, rectum and anus. Part I. Clinical classification. Jpn J Surg. 1983. 13:557–573.
7. Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. 2002. 6th ed. New York: Wiley-Liss.
8. Kirklin JW, Dockerty MB, Waugh JM. The role of the peritoneal reflection in the prognosis of carcinoma of the rectum and sigmoid colon. Surg Gynecol Obstet. 1949. 88:326–331.
9. Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954. 139:846–852.
10. Gabriel WB, Dukes C, Bussey HJR. Lymphatic spread in cancer of the rectum. Br J Surg. 1935. 23:395–413.
11. Dukes CE, Bussey HJ. The spread of rectal cancer and its effect on prognosis. Br J Cancer. 1958. 12:309–320.
12. Sternberg A, Sibirsky O, Cohen D, Blumenson LE, Rodriguez-Bigas MA, Petrelli NJ. New approach to the substaging of node-positive colorectal adenocarcinoma. Ann Surg Oncol. 1999. 6:161–165.
13. Greene FL, Page DL, Fleming ID. AJCC Cancer Staging Manual. 2002. 6th ed. New York: Springer.
14. Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000. 124:979–994.
15. Maurel J, Launoy G, Grosclaude P, Gignoux M, Arveux P, Mathieu-Daude H, et al. Lymph node harvest reporting in patients with carcinoma of the large bowel: a French population-based study. Cancer. 1998. 82:1482–1486.
16. Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol. 2002. 26:179–189.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download