Abstract
Purpose
The aim of this study is to present cases of postoperative leakage after various types of gastrointestinal operations that were successfully managed with fluoroscopy-guided Foley catheter.
Methods
Fluoroscopy-guided Foley catheter insertion and drainage methods were performed in 13 leakage sites of 10 patients diagnosed as having leakage after gastrointestinal operations such as esophagectomy, gastrectomy and appendectomy. Under fluoroscopic guidance, a guide-wire was inserted into the leakage site where a Foley catheter was then introduced over the guide wire, inserted and ballooned.
Results
The median time for the procedures was 30 minutes (range: 20~260 minutes), with esophagus or stomach leakage sites requiring a longer procedure time than the appendiceal or duodenal stump. The indwelling Foley catheters were successfully removed after a median of 11 days (range: 8~44 days), and the opening of the enterocutaneous fistulas were spontaneously closed in eight out of 10 patients.
Leakage after gastrointestinal anastomosis is a challenging complication. The rate of anastomotic disruption has been known to range from 0.5 to 30%,(1,2) and many researchers have reported that several technical factors and patients' conditions were related to its occurrence.(3,4) Recent developments in surgical technique, as well as the development of improved postoperative care, have decreased the occurrence rate of leakage. However, this problem can still burden surgeons owing to the potential sequela, which include fluid collection, abscess, hemorrhage, malnutrition and sepsis. In addition, it may lead to fatal complications in these patients; a study reported that the leakage-related mortality rate varied from 4.8 to 75% according to the leakage sites, where partial gastrectomy had the highest rate.(5) Recently, leakage management has been developed towards reducing hospitalized days, morbidity and mortality. In particular, patients who present localized peritonitis after leakage are generally being treated with a percutaneous simple drainage method, even though this does not satisfy physicians due to inconvenience to patients and the length of time spent for complete cessation of the leakage. More recently, although glue applied to the fistula tract or stent that covers the leakage site under endoscopy has also been tried, there were no confirmative results.(6-9)
Thus, we tried a new treatment modality for patients with postoperative anastomotic leakage that was not indicated for the surgical intervention. Our plan was to turn an anastomotic leakage into a well-controlled fistula as soon as possible by inserting a Foley catheter through the leakage site. In this report, we present a series of cases of postoperative leakage after various types of gastrointestinal operations that were successfully managed with fluoroscopy-guided Foley catheter insertion and drainage.
From December 2005 to July 2007, fluoroscopy-guided Foley catheter insertion and drainage was performed in 10 patients who were diagnosed with postoperative leakage after gastrointestinal surgery at the Department of Surgery, St. Mary's Hospital. The leakages had developed in five patients after gastric resection for gastric cancer, in two patients after appendectomy for perforated appendicitis, in one patient after antrectomy for a perforated duodenal ulcer, in one patient after transhiatal esophagectomy for esophageal cancer and in one patient after distal pancreatosplenectomy with proximal gastrectomy for pancreatic body cancer that involved the posterior wall of the upper stomach. The procedures were performed at postoperative day 4 to 130 (median: 16) (Table 1). We enrolled the patients who presented localized tenderness without generalized peritonitis signs, as imaging studies found the localized abscess. Using gastrograffin swallowing or tubogram tests, we selected patients in whom the bowel content from the point of leakage did not spread over the whole abdominal cavity. Mainly, the patients with leakage sites which were possible to approach by interventional method were included in this study. All patients provided informed written consent for this procedure.
Before the procedure, patients were supine on the fluoroscopy table. The guide catheter, of various types, was inserted through the indwelling drain to obtain a tubogram (Fig. 1A). In patients who did not have external drainage of leaked content or who failed to obtain a proper tubogram, the gastrograffin swallowing test was performed immediately prior to the procedure, and a guide catheter was then percutaneously inserted near the site of leakage. Under fluoroscopic guidance, a 0.035-inch Terumo hydrophilic guide wire (Terumo, Tokyo, Japan) was introduced through the guide catheter (Fig. 1B). The fluoroscopic examination allowed confirmation of correct placement of the guide wire. After the guide-wire was precisely placed in the bowel lumen, a Foley catheter (Sewoon Medical, Seoul, Korea) sized from 8 to 16 French was inserted over the guide-wire and ballooned (Fig. 1C). Although the type of Foley catheter and the size of ballooning were decided according to the features of the leakage site and bowel lumen, 8 to 12 French and 6 to 8 cc ballooning commonly used. We confirmed catheter placement by tubogram through the Foley catheter (Fig. 1D). Finally, the catheter was anchored with mild tension (Fig. 2). Interestingly, we had difficulty in percutaneously approaching the leakage site of esophagogastric anastomosis in the lateral neck while performing the procedure in patients with transhiatal esophagectomy. Therefore, we introduced the guide-wire through the oral cavity to the leakage site in the neck, snared the wire with a percutaneously introduced stone retrieval basket and inserted the Foley catheter in the inner side of the lumen.
After the procedure, we observed patients overnight without oral intake for whether the drainage was effective and whether they showed symptoms of peritonitis. On the next day, patients began with a sip of water and, if that was tolerable, advanced to their regular diet and normal physical activity on the second day after the procedure. The wound dressing for the catheter insertion site was changed once a day if drainage of bowel contents through the catheter was complete. If there was no complaint of fever or abdominal pain on the third day after the procedure, the catheter could be locked. After observation for about more two days in the hospital, a discharge was recommended under normal conditions. In ambulatory care, the ballooning was withdrawn and the catheter was removed unless the patients presented symptom or the leakage of contents around fistula.
For the 13 leakage sites in 10 patients, all management trials were successfully performed by inserting a Foley catheter into the bowel lumen through leakage sites (Table 1). There were no complications during procedures, and the median procedure time was 60 minutes (range: 20~260 minutes) and differed according to leakage site: duodenal stump, 35.8 minutes (20~60 minutes); appendiceal stump, 45 minutes (30~60 minutes); esophagus and stomach, 156 minutes (120~260 minutes).
Finally, 10 (76.9%) of the 13 leakage sites were successfully managed by this procedure. At a median of 11 days after the procedure (range: 8~44 days), we could remove the Foley catheter. Among three sites that failed to manage the leakage, two leakage sites in one patient who underwent total gastrectomy could not be managed owing to failure of fixation of the Foley catheter. The other site was a duodenal stump that was made after a distal gastrectomy, and this patient had to be re-operated because of bowel contents discharged from the removal site for more than 2 months. The re-operative findings showed a short length of the fistula tract.
Once an anastomotic leakage is suspected or diagnosed, immediate resuscitation should be started, including the removal of the intra-abdominal collection of the intestinal contents, suspension of oral intake, supply of intravenous fluids and injection of antibiotics.(10) However, leakage management depends on the clinical status of patient's condition and the location of the leakage site. When there is diffuse peritonitis, intra-abdominal hemorrhage or major wound disruption, re-operation should be considered, which can be associated with serious complications due to the invasive nature of surgery and the poor general condition of these patients. Meanwhile, an intra-abdominal abscess with locally confined peritonitis after leakage can be managed with percutaneous drainage because localized peritonitis is considered as small amounts of leaked content following the partial disruptions of suture line.(11)
In the percutaneous simple drainage method, the tip of the drainage catheter would be located near the leakage site, and a fistula tract would be made around the catheter and close spontaneously within two to six weeks with proper management if drainage is effective.(12) However, to ensure effective drainage and wound healing, oral intake should not be allowed in order to decrease the amount of bowel contents released from the leakage site. This release could lead to longer hospitalization accompanied by a higher cost for treatments. In addition, persistence of septic conditions due to insufficient leakage site control could make the patient's quality of life progressively deteriorate. Eventually, a surgical procedure may be required, which can be associated with increased morbidity and mortality.
Although the principle of drainage using Foley catheter insertion is similar to simple drainage techniques with respect to using interventional radiology without reoperation, our method has several clinical advantages over simple drainage techniques, as follows: First, the proper size of the ballooning could obstruct the leakage site, and it is easy to prevent local fluid collection and take care of the skin around the external os., if the catheter can maintain the tension. Also, it is possible to form the fistula tract faster than in a simple procedure. Second, effective drainage through the catheter allowed the patients to orally intake their nutrition. Although the selection of a nutritional support route is generally dependent on the leakage site, enteral nutrition is the safest and most effective method.(13,14) Finally, this method allows reduced hospitalized days and admission cost.
The technique adopted in our study was previously used in fluoroscopically guided percutaneous gastrostomy with a large-bore balloon-retained catheter.(15,16) Chan et al.(15) reported that this technique did not require surgery and was an effective procedure to supply enteral nutrition without a failed case or major complication. However, several minor complications, such as tube dysfunction and ballooning rupture during the procedure, were reported. In our results, although all cases were performed successfully without morbidity and mortality during the procedure, there was some difference in the procedure time or difficulty of approach. Leakage sites of duodenal and appendiceal stumps were easy to approach, and it took less than 60 minutes to perform these procedures because the areas are partially fixed to the retroperitoneum, which easily led the guide-wire to the leakage point. However, for a leakage site such as esophago-jejunostomy after total gastrectomy or gastrotomy for end to end anastomotic device (EEA) insertion, which are located in a deep portion of the abdominal cavity and not fixed, it took a longer time to find the leakage site under fluoroscopy.
Moreover, three sites that failed to close after successful insertion of the catheter let us know the relative contra-indications for this procedure. In two of the three sites, related to total gastrectomy, it was difficult to fix the Foley into the lumen, and ballooning easily escaped from the lumen. On the other hand, a duodenal stump showed a persistent enterocutaneous fistula for 2 months after the removal of Foley catheter. We could confirm the short tract in the later re-operation field. Therefore, a difficult area in which to fix the Foley or a short distance from skin to leakage site might have difficult to apply our procedure.
In conclusion, the application of fluoroscopy-guided Foley catheter insertion and drainage on the control of leakage sites of anastomosis or suture line showed good results in a short hospital stay, with the possibility of outpatient follow up, maintenance of oral intake and normal activity, effective control of the leakage site and protection of the skin from intestinal contents. Therefore, this method is a useful strategy to control anastomotic leakage.
Figures and Tables
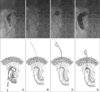 | Fig. 1Fluoroscopic findings and illustration of Foley catheter insertion into the site of leakage. (A) Leakage confirmation. (B) The introduction of guide wire through the guide catheter. (C) The insertion and ballooning of the Foley catheter through the guide wire. (D) The confirmation of placement of the Foley catheter by tubogram. |
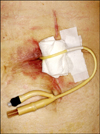 | Fig. 2Inserted Foley catheter into leaking site and drainage of leaked content in a patient with leakage in the duodenal stump after distal gastrectomy. |
Table 1
Leakage site and results of procedures in patients with fluoroscopic Foley catheter insertion and drainage

*POD = postoperative day; †Fr = french; ‡PPD = postprocedure day; §LADG = laparoscopy-assisted distal gastrectomy; ∥B-II = Billroth-II; ¶ODG = open distal gastrectomy; **EEA = end to end anastomosis; ††Revision of the leakage site and re-anastomosis owing to frequent escape of Foley ballooning; ‡‡Revision of the duodenal stump and re-closure due to failure of spontaneous closure of the enterocutaneous fistula due to short tract; §§PG = proximal gastrectomy.
References
1. Fielding LP, Stewart-Brown S, Blesovsky L, Kearney G. Anastomotic integrity after operations for large-bowel cancer: a multicentre study. Br Med J. 1980. 281:411–414.
2. Tuson JR, Everett WG. A retrospective study of colostomies, leaks and strictures after colorectal anastomosis. Int J Colorectal Dis. 1990. 5:44–48.
3. Golub R, Golub RW, Cantu R Jr, Stein HD. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg. 1997. 184:364–372.
4. McIntyre PB, Ritchie JK, Hawley PR, Bartram CI, Lennard-Jones JE. Management of enterocutaneous fistulas: a review of 132 cases. Br J Surg. 1984. 71:293–296.
5. Pickleman J, Watson W, Cunningham J, Fisher SG, Gamelli R. The failed gastrointestinal anastomosis: an inevitable catastrophe? J Am Coll Surg. 1999. 188:473–482.
6. Cho YP, Lee DH, Jang HJ, Kim JS, Kim YH, Han MS, et al. Leakage of jejunal end of roux limb after total gastrectomy: management with a placement of a covered metallic stent: case report. J Korean Med Sci. 2003. 18:437–440.
7. Kwak HS, Lee JM, Jin GY, Han YM, Yang DH. Treatment of gastrojejunal anastomotic leak with a covered metallic stent. Hepatogastroenterology. 2003. 50:62–64.
8. Papavramidis ST, Eleftheriadis EE, Papavramidis TS, Kotzampassi KE, Gamvros OG. Endoscopic management of gastrocutaneous fistula after bariatric surgery by using a fibrin sealant. Gastrointest Endosc. 2004. 59:296–300.
9. Truong S, Bohm G, Klinge U, Stumpf M, Schumpelick V. Results after endoscopic treatment of postoperative upper gastrointestinal fistulas and leaks using combined Vicryl plug and fibrin glue. Surg Endosc. 2004. 18:1105–1108.
10. Li J, Ren J, Zhu W, Yin L, Han J. Management of enterocutaneous fistulas: 30-year clinical experience. Chin Med J (Engl). 2003. 116:171–175.
11. Reid-Lombardo KM, Farnell MB, Crippa S, Barnett M, Maupin G, Bassi C, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy in 1,507 patients: a report from the Pancreatic Anastomotic Leak Study Group. J Gastrointest Surg. 2007. 11:1451–1458. discussion 9.
12. Papanicolaou N, Mueller PR, Ferrucci JT Jr, Dawson SL, Johnson RD, Simeone JF, et al. Abscess-fistula association: radiologic recognition and percutaneous management. AJR Am J Roentgenol. 1984. 143:811–815.
13. Baigrie RJ, Devitt PG, Watkin DS. Enteral versus parenteral nutrition after oesophagogastric surgery: a prospective randomized comparison. Aust N Z J Surg. 1996. 66:668–670.
14. Meguid MM, Campos AC. Nutritional management of patients with gastrointestinal fistulas. Surg Clin North Am. 1996. 76:1035–1080.
15. Chan SC, Ko SF, Ng SH, Cheung YC, Chang JT, Liao CT, et al. Fluoroscopically guided percutaneous gastrostomy with modified gastropexy and a large-bore balloon-retained catheter in patients with head and neck tumors. Acta Radiol. 2004. 45:130–135.
16. Hicks ME, Surratt RS, Picus D, Marx MV, Lang EV. Fluoroscopically guided percutaneous gastrostomy and gastroenterostomy: analysis of 158 consecutive cases. AJR Am J Roentgenol. 1990. 154:725–728.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download