Abstract
Purpose
COX-2 is known to be elevated in breast cancer, but the clinical relevance is still a matter of debate. The purpose of this study was to determine the prognostic significance and relationship of COX-2 to hormone receptors.
Methods
Between January 2005 and February 2007, 80 specimens from breast cancer patients at Korea University Anam Hospital were reviewed by one pathologist. COX-2 was analyzed as overexpressed if >10% of the cells were stained. Clinical characteristics, hormone receptor status, and other prognostic factors were investigated to determine their association with COX-2 expression.
Results
COX-2 was overexpressed in 12 patients (15%). Two patients had locoregional recurrence, eight patients had systemic metastasis, and one patient died. There was no statistically significant correlation between COX-2 expression and age, size, nodal status, histological grade, hormone receptor status, and HER-2/neu positivity. Among tumors that had a positive expression of ER and PR, COX-2 expression was related to larger size (P-value 0.001 and 0.009, respectively) and nodal status (P-value 0.048 and 0.009, respectively). However, there was no statistically significant correlation with tumors that had negative ER or PR expression.
Conclusion
This study suggests that in breast cancer, COX-2 expression has no relationship with clinicopathologic factors; however, a correlation was noted in size and nodal status for ER- and PR-positive tumors. Further prospective study with larger population to clarify the relationship between COX-2 expression and hormone receptor status is necessary.
Estrogen is an important factor in the etiology of breast cancer. It is regulated by the cytochrome P-450 enzyme complex known as aromatase, which catalyzes androgen to produce estrogen. Cyclooxygenase-2 (COX-2) plays a role in the regulation of estrogen since it produces prostaglandin E2, which increases the expression of the cytochrome P-450 enzyme complex. Prostaglandin E2 is produced not only by COX-2, but by COX-1. However, COX-1 is constitutively produced by most tissues, while COX-2 is induced in the environment with mitogens, cytokines, hormones, and serum. Molecular studies suggest that COX-2 is related to mutagenesis, angiogenesis, inhibition of apoptosis, and aromatase-catalyzed estrogen biosynthesis; there is also a hypothesis that local estrogen levels induced by elevated aromatase activity stimulate tumor growth, thus the development and role of COX-2 seems to be essential in breast cancer.(1-5)
COX-2 is known to be expressed in several cancers, including colorectal, prostate, lung, pancreas, and breast. (6-11) Moreover, many studies suggest that COX-2 is a prognostic factor for various cancers, especially colorectal adenocarcinomas.(8) Based on these theories, targeting COX-2 is thought to be an effective strategy for treatment, which has given rise to clinical trials with COX-2 inhibitors, such as celecoxib and rofecoxib for colorectal cancer.(12)
COX-2 selective inhibitors suppress tumorigenesis in rat models of breast cancer,(13) but unlike colorectal cancer, studies relating COX-2 to breast cancer have not shown consistency with respect to clinicopathologic and prognostic significance. Although estrogen is clearly related to COX-2, the relationship between COX-2 and the estrogen receptor (ER) for breast cancer is unclear. The aim of this study was to investigate the prognostic significance of COX-2 and its relationship to the hormone receptors.
We identified 80 patients who were diagnosed with breast cancer at Korea University Anam Hospital between January 2005 and February 2007; their specimens were collected retrospectively. The patients were all females who were diagnosed with invasive ductal carcinoma by light microscopy using conventional hematoxylin and eosin stain, and underwent modified radical mastectomy or breast-conserving surgery.
The baseline patient characteristics are summarized in Table 1. Among 80 patients, 57 patients underwent axillary node dissection, while 23 patients had negative results on sentinel lymph node biopsy. Patients who did not have surgical treatment were excluded. Adjuvant chemotherapy was administered according to the pathologic report and all patients who underwent breast-conserving surgery received radiation therapy and those who were hormone receptor-positive had adjuvant hormonal therapy.
Size and nodal status were divided using the criteria of the TNM system. Histological grade was evaluated using the Bloom and Richardson criteria as guidelines suggested by the Nottingham City Hospital Pathologists.(14)
Archived paraffin-embedded tissue samples were studied. Two consecutive 4µm-thick sections were cut from the paraffin-embedded block. One section from the specimen was routinely stained with hematoxylin-and-eosin. Another section was immunohistochemically stained for COX-2.
Tissue sections were deparaffinized in xylene, and rehydrated with a graded series of ethanol to water. The activity of peroxidase was inhibited by precipitation in methanol with 3% H2O2 for 5 minutes. The slides were cooked with Tris-EDTA buffer (pH 9.0) in an autoclave at 121℃ for 5 minutes for COX-2. Samples were incubated with COX-2 antibody (1:100; Dako, Copenhagen, Denmark) at room temperature for 30 minutes. After washing, the sections were incubated with a secondary antibody (ChemMate DAKO Envision) at room temperature for 30 minutes. Subsequently, the sections were subjected to DAB (substrate buffer+DAB chromogen [X50]) for 5 minutes. The slides were counterstained with hematoxylin and then mounted.
COX-2 cytoplasmic evaluation was made by intensity scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong) and percentage of positive tumor cells. COX-2 was considered overexpressed when the intensity was scored with 2 and 3 with more than 10% of positive tumor cells (Fig. 1).
Assessment of ER and progesterone receptor (PR) status was done by standard immunohistochemical methods and considered positive if the value was >10% of nuclear staining. Evaluation was done by single pathologist using a light microscope.
Comparison of clinicopathologic factors between COX-2 overexpressed and unexpressed patients were assessed using SPSS, version 12. Chi-square tests were used for univariate analysis, and logistic regression with forward procedure was used for statistically significant factors in multivariate analysis. For comparison of disease-free survival, Kaplan-Meier survival curves were used. Statistical significance was regarded when the P-value was <0.05.
The correlation between clinicopathologic factors and COX-2 expression of 80 patients are summarized in Table 2. There were 12 patients with overexpressed COX-2 and 68 patients with unexpressed COX-2, with a positivity rate of 15%.
No statistical significance was found between COX-2 overexpressed patients and COX-2 unexpressed patients with respect to age, tumor size, nodal status, and hormone receptors status.
The mean follow-up period was 34.125±8.266 months. Eight patients developed distant metastasis (two patients in the liver and bone, three patients in bone only, two patients in the lungs, and one patient in the brain). One patient had breast recurrence and one patient died.
We divided the group into ER-positive and -negative groups to compare the differences in clinicopathologic factors between COX-2-overexpressed and -unexpressed patients in each group. Comparison of the ER and PR group is outlined in Table 3 and 4. COX-2 overexpression was more common in larger tumors and higher nodal status with P-values of <0.001 and 0.048, respectively. Larger size and higher nodal status was more commonly positive for COX-2 overexpression patients in the PR-positive group (Table 4) with a P-value of 0.009. However in multivariate analysis, no correlation was found between clinicopathologic parameters and COX-2 expression. There were no statistically significant factors in the ER-negative group and PR-negative group.
Many factors are known to be prognostic factors for breast cancers, including size, nodal status, hormone receptors, and HER2/neu expression. For targeting treatment, hormone therapy and targeting HER2/neu have been successful and they have become the mainstay of treatment of breast cancer patients along with chemotherapy and radiation therapy. Adding to these treatment strategies, different factors are being studied, one of which is COX-2. Brueggemeier et al.(1,2) showed the regulation of estrogen in relation to COX-2, and concluded that elevation of COX-2 expression results in increased aromatase activity via autocrine and paracrine mechanisms which underlie the pathogenesis of breast cancer. Although there are theories involving the relationship between COX-2 and breast cancer, clinical relevance or prognostic values are still controversial, thus we attempted to determine the relationship between clinicopathologic factors and COX-2 expression.
The positivity of COX-2 in breast cancer varies from 4.5% to 85%,(6,15) and in our study 15% of all invasive ductal cancers had an elevated expression of COX-2. The reason for variation in the positivity of COX-2 appears to be because of an unclear definition and inconsistency of increased COX-2 in most of the studies. Cho et al.(16) determined COX-2 by multiplying the staining intensity score and staining quantity score, while Ristimaki et al.(6) and Haffty et al.(17) considered COX-2 moderately-to-strongly positive when >10% was stained in cancer cells, which was how we evaluated COX-2 positivity herein.
This study was conducted to determine the significance of COX-2 in breast cancer, but we found no association between clinicopathologic factors, including age, tumor size, nodal status, histological grade, hormone receptor status, and COX-2 expression. We also attempted to determine a relationship with other prognostic factors, such as ki-67 and p-53, but there was no statistical significance; this result was consistent with other studies. Kelly et al.(15) studied 106 breast cancer patients and found no statistical correlation between COX-2 expression level and other prognostic indicators, such as node status, tumor size, histological grade, histology, and ER or PR status. Thorat et al.(18) tried to find a relationship between COX-2 expression and microvessel density in breast cancer. The histological grade and ER status were the only factors to correlate with COX-2 status.(18) However, in Finland, Ristimaki et al.(6) conducted a multicenter study with a large population of 1,576 patients with invasive ductal breast carcinoma, concluding that COX-2 overexpression was related to poor prognosis with reduced survival. Unlike other studies, Denkert et al.(19) examined both isoforms of cyclooxygenase with 221 primary breast cancer patients. COX-1 and COX-2 were overexpressed in 45.5% and 36.2% of all patients, respectively. COX-1 is known to be expressed in most normal tissues, and likewise only COX-2 expression was associated with prognosis and tumor size, histological grade, nodal status, and angioinvasion. In previous animal studies, celecoxib, a COX-2 inhibitor, was reported to be more effective in reducing tumor development and volume than ibuprofen, a non-specific cyclooxygenase inhibitor,(20-22) which was consistent with the report of Denkert et al.(19) regarding the prognosis. Inconsistent results of relationship between COX-2 expression and other clinicopathological factors or prognosis remains undetermined. However, it could be explained by lack of clarified mechanism of increased COX-2 expression in breast cancer and subjective evaluation of COX-2 staining. Although COX-2 expression was not associated with clinicopathological factors in our study, it would be interesting to conduct a well-designed study with a larger population and well-developed objective definition of COX-2 expression to further determine the effectiveness of COX-2 inhibitors for breast cancer therapy and prevention.
Ristimaki et al.(6) not only compared the prognostic factors with COX-2 expression, but they compared clinical factors in different subgroups of patients. Patients with a positive ER tend to derive more prognostic value with COX-2 than the ER-negative group, but they did not have results for correlation with clinical factors. We compared clinicopathologic factors in ER, PR positive and negative groups. Larger size and higher nodal status was related to COX-2 positivity in the ER-positive group and the same was found with the PR-positive group, while there were no significant factors in the ER/PR negative groups.
Survival curves, except for disease-free survival, could not be evaluated in this study because of the short follow-up period. There were no significant differences in disease-free survival. Since COX-2 regulates estrogen in an autocrine or paracrine pathway, there appears to be a relationship with receptor status. Ristimaki et al.(6) suggested that elevated COX-2 expression in ER-positive cancers could enhance a microenvironment for cancer cells to grow by inducing estrogen production. Haffty et al.(17) reported a correlation between COX-2 expression and younger age, tumor size, higher breast relapse rates, more distant metastases, and mortality. They also reported that in the ER-positive group there was a stronger association with prognosis than in the ER-negative group. Although there are controversies of prognostic relevance of COX-2 expression, preclinical studies suggest the interconnection between aromatase and COX-2 pathways.(1,2,5,23) Based on these reports, several clinical trials investigated combination therapy with aromatase inhibitor (AI) and COX-2 inhibitor. The Celecoxib Anti-Aromatase Neoadjuvant (CAAN) trial treated 82 postmenopausal advanced breast cancer patients in three groups, exemestane and celecoxib, exemestane alone and letrozole alone for 3 months before surgery. The preliminary report shows overall response rate was 58.6% for combination therapy, 54.5% for exemestane alone group and 62% for letrozole alone group. They suggested that COX-2 inhibitor contribution is yet to be determined since they are still in initial phase of study.(24) Falandry et al.(23) designed a study comparing treatment between exemestane with celecoxib and exemestane with placebo for postmenopausal metastatic breast cancer patients resistant to tamoxifen therapy. The study started as a multicenter double-blinded randomized phase III trial involving 62 sites in France but terminated early after other trials with celecoxib reported cardiovascular toxicity. However, with 157 patients, they reported longer progression-free survival with tamoxifen-resistant patients who were treated with exemestane and celecoxib than with placebo (8.4 vs 4.7 months).(1-4) As mentioned earlier, the mechanism has not been distinct but there are studies suggesting the interconnection(5) and there are ongoing clinical trials for verification.(23)
The relationship between HER-2/neu and COX-2 has also been established. Several reports have concluded that HER-2/neu overexpression increase COX-2 via the Raspathway.(25-27) Howe et al.(27) reported that when there is COX-2 deficiency in HER2/neu highly expressed breast cancer, tumor formation and growth decreases and this was shown with COX-2 knock-out mice. These results may lead us to use COX-2 inhibitor as a prevention of breast cancer with HER2/neu-overexpressed cancer. However, this study suggests that there is an inverse relationship between HER2/neu expression and COX-2 positivity, although there was no significance.
The absence of a relationship with COX-2 and clinicopathologic factors was inconclusive since the population was small, with 80 invasive ductal carcinoma tissues, and may have selection bias as it was a retrospective study. Also, there is yet no agreement concerning the definition of COX-2 positivity and we think this might be a priority matter internationally.
In conclusion, we identified no correlation between clinicopathologic factors and COX-2 expression, but size and nodal status appear to be related to COX-2 positivity in the ER-positive group and controversies still remain for the clinical significance of COX-2 expression.
Figures and Tables
Fig. 1
Cox-2 immunohistochemical staining in invasive ductal breast carcinoma. (A) Negative immunoreactivity (H&E stain, ×400). (B) Positive immunoreactivity in cytoplasm of >10% of tumor cells (H&E stain, ×400).
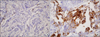
Fig. 3
Disease-free survival (DFS) comparison by hormone receptor status. (A) ER positive group. (B) ER negative group. (C) PR negative group. Survival curve couldn't be drawn in PR positive group because of small number of events.

References
1. Brueggemeier RW, Diaz-Cruz ES, Li PK, Sugimoto Y, Lin YC, Shapiro CL. Translational studies on aromatase, cyclooxygenases, and enzyme inhibitors in breast cancer. J Steroid Biochem Mol Biol. 2005. 95:129–136.
2. Brueggemeier RW, Richards JA, Petrel TA. Aromatase and cyclooxygenases: enzymes in breast cancer. J Steroid Biochem Mol Biol. 2003. 86:501–507.
3. Diaz-Cruz ES, Shapiro CL, Brueggemeier RW. Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab. 2005. 90:2563–2570.
4. Richards JA, Petrel TA, Brueggemeier RW. Signaling pathways regulating aromatase and cyclooxygenases in normal and malignant breast cells. J Steroid Biochem Mol Biol. 2002. 80:203–212.
5. Brodie AM, Lu Q, Long BJ, Fulton A, Chen T, Macpherson N, et al. Aromatase and COX-2 expression in human breast cancers. J Steroid Biochem Mol Biol. 2001. 79:41–47.
6. Ristimaki A, Sivula A, Lundin J, Lundin M, Salminen T, Haglund C, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002. 62:632–635.
7. Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, et al. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001. 91:333–338.
8. Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995. 55:3785–3789.
9. Tjandrawinata RR, Dahiya R, Hughes-Fulford M. Induction of cyclo-oxygenase-2 mRNA by prostaglandin E2 in human prostatic carcinoma cells. Br J Cancer. 1997. 75:1111–1118.
10. Castelao JE, Bart RD 3rd, DiPerna CA, Sievers EM, Bremner RM. Lung cancer and cyclooxygenase-2. Ann Thorac Surg. 2003. 76:1327–1335.
11. Jang HR, Yang KH, Bae BN, Kim KH, Han SH, Kim HJ, et al. The clinical significance of cyclooxygenase 2 expression in colorectal cancer. J Korean Surg Soc. 2003. 64:39–43.
12. Buecher B, Bouancheau D, Broquet A, Bezieau S, Denis MG, Bonnet C, et al. Growth inhibitory effect of celecoxib and rofecoxib on human colorectal carcinoma cell lines. Anticancer Res. 2005. 25:225–233.
13. Connolly EM, Harmey JH, O'Grady T, Foley D, Roche-Nagle G, Kay E, et al. Cyclo-oxygenase inhibition reduces tumour growth and metastasis in an orthotopic model of breast cancer. Br J Cancer. 2002. 87:231–237.
14. Robbins P, Pinder S, de Klerk N, Dawkins H, Harvey J, Sterrett G, et al. Histological grading of breast carcinomas: a study of interobserver agreement. Hum Pathol. 1995. 26:873–879.
15. Kelly LM, Hill AD, Kennedy S, Connolly EM, Ramanath R, Teh S, et al. Lack of prognostic effect of Cox-2 expression in primary breast cancer on short-term follow-up. Eur J Surg Oncol. 2003. 29:707–710.
16. Cho MH, Yoon JH, Jaegal YJ, Choi YD, Lee JS, Lee JH, et al. Expression of cyclooxygenase-2 in breast carcinogenesis and its relation to HER-2/neu and p53 protein expression in invasive ductal carcinoma. Breast. 2006. 15:390–398.
17. Haffty BG, Yang Q, Moran MS, Tan AR, Reiss M. Estrogen-dependent prognostic significance of cyclooxygenase-2 expression in early-stage invasive breast cancers treated with breast-conserving surgery and radiation. Int J Radiat Oncol Biol Phys. 2008. 71:1006–1013.
18. Thorat MA, Mehrotra S, Morimiya A, Badve S. COX-2 expression does not correlate with microvessel density in breast cancer. Pathobiology. 2009. 76:39–44.
19. Denkert C, Winzer KJ, Muller BM, Weichert W, Pest S, Kobel M, et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003. 97:2978–2987.
20. McCormick DL, Moon RC. Inhibition of mammary carcinogenesis by flurbiprofen, a non-steroidal antiinflammatory agent. Br J Cancer. 1983. 48:859–861.
21. Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000. 60:2101–2103.
22. Alshafie GA, Abou-Issa HM, Seibert K, Harris RE. Chemotherapeutic evaluation of Celecoxib, a cyclooxygenase-2 inhibitor, in a rat mammary tumor model. Oncol Rep. 2000. 7:1377–1381.
23. Falandry C, Canney PA, Freyer G, Dirix LY. Role of combination therapy with aromatase and cyclooxygenase-2 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2009. 20:615–620.
24. Chow LW, Yip AY, Loo WT, Lam CK, Toi M. Celecoxib anti-aromatase neoadjuvant (CAAN) trial for locally advanced breast cancer. J Steroid Biochem Mol Biol. 2008. 111:13–17.
25. Benoit V, Relic B, Leval Xd X, Chariot A, Merville MP, Bours V. Regulation of HER-2 oncogene expression by cyclooxygenase-2 and prostaglandin E2. Oncogene. 2004. 23:1631–1635.
26. Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in HER-2/neu-positive breast cancer: evidence for involvement of AP-1 and PEA3. J Biol Chem. 2002. 277:18649–18657.
27. Howe LR, Chang SH, Tolle KC, Dillon R, Young LJ, Cardiff RD, et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005. 65:10113–10119.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download