Abstract
Purpose
Tacrolimus (FK506) has been widely used as an immunosuppressant in organ transplanted recipients to suppress organ rejection phenomenon. We investigated the role of oxidative stress and heme oxygense-1 by FK506 on human Jurkat T cells.
Methods
The cells viability was examined by DAPI stain, enzyme activity of caspase family proteins, and western blotting for Baks, PUMA, iNOS, HO-1. Cells were cultured in the absence or presence of CoPPIX or ZnPPIX and the fluorescence intensity was analyzed using a flow cytometry.
Results
Treatment with FK506 increased the generation of reactive oxygen species (ROS), including hydrogen peroxide and superoxide anion, and NO in Jurkat cells in a dose-dependent manner. Immunohistochemistry and Western blot analysis data revealed the hemoxygenase-1 (HO-1) was induced by the addition of FK506 in Jurkat cells. Induction of CoPP, HO-1 inducer, resulted in decreased intracellular H2O2 and NO concentrations. Instead ZnPP, an HO-1 competitive inhibitor did it reversely. In addition, ZnPP regulates iNOS protein synthesis by inhibition of HO-1.
Conclusion
Increase of HO-1 expression would induce to decrease the intracellular H2O2 and NO concentrations. Also, HO-1 would regulate iNOS protein synthesis. Consequently, we can expect the regulation of HO-1 expression with concomitants use of FK506 to suppress organ rejection phenomenon by enhancing apoptosis.
Figures and Tables
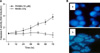 | Fig. 1FK506 induced cytotocixity and nuclear fragment on Jurkat cells. (A) Cells were treated with 10 µM FK506 for 12 to 72 hr and lysed to measure the activity of caspase proteases by using fluorogenic biosubstrates. Data represent the mean±standard deviation (S.D.) of quadruplicates. (B) Cells were treated with FK506 (10 µM) for 72 hr. Then, cells were stained with DAPI and observed under fluorescence microscopy. |
 | Fig. 2Production of H2O2 in FK506 treated Jurkat cells. Cells were treated with indicated dose of FK506 for 72 hrs. Then, cells were incubated with the dye 2', 7'-dichlorofluorescin diacetate (5 µM) and the fluorescence intensity of more than 10,000 cells was analyzed using a flow cytometry. |
 | Fig. 3Effects of NO production and iNOS protein expression in Jurkat cells. (A) Cells were treated with indicated dose of FK506 for 72 hrs. Then, cells were incubated with the dye DAF-DA (5 µM) and the fluorescence intensity of more than 10,000 cells was analyzed using a flow cytometry. (B) Histogram status of A Cells were treated with 10 µM FK506 for various periods. The equal amounts of protein from cell lysate were subjected on 10% SDS-PAGE, transferred onto nitrocellulose membrane and immunoblotted with anti-iNOS and anti-β-actin antibodies. |
 | Fig. 4Change of mitochondrial membrane potential transition and differential expression of Bak and PUMA in FK506 treated Jurkat cells. (A) Cells were treated with 10 µM FK506 for 36 hr. FK506 treated cells were stained with 10 µg/ml of JC-1 visualized under a fluorescent microscope. (a) Control cells, and (b) FK506 treated cells for 36 hrs. (B) Cells were treated with 10 µM FK506 for various periods. The equal amounts of protein from cell lysate were subjected on 15% SDS-PAGE, transferred onto nitrocellulose membrane and immunoblotted with PUMA, anti-Bak and anti-β-actin antibodies. |
 | Fig. 5Effects of H2O2 production and HO-1 protein expression in Jurkat cells by CoPP or ZnPP. (A) Cells were cultured in the absence or presence of CoPP or ZnPP for 48 hrs. Cells were treated with 10 µM FK506 for various periods. Then, cells were incubated with the dye DCF-DA (5 µM) and the fluorescence intensity of more than 10,000 cells was analyzed using a flow cytometry. (B) Histogram status of A. (C) The equal amounts of protein from cell lysate were subjected on 10% SDS-PAGE, transferred onto nitrocellulose membrane and immunoblotted with anti-HO-1 and anti-β-actin antibodies. The immunoreactive signals were visualized by ECL detection kit. |
 | Fig. 6Effects of NO production and iNOS protein expression in Jurkat cells by CoPP or ZnPP. (A) Cells were cultured in the absence or presence of CoPP or ZnPP for 48 hrs. Cells were treated with 10 µM FK506 for various periods. Then, cells were incubated with the dye DAF-DA (5 µM) and the fluorescence intensity of more than 10,000 cells was analyzed using a flow cytometry. (B) Histogram status of A. (C) The equal amounts of protein from cell lysate were subjected on 10% SDS-PAGE, transferred onto nitrocellulose membrane and immunoblotted with anti-iNOS and anti-β-actin antibodies. The immunoreactive signals were visualized by ECL detection kit. |
References
1. Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS. FK506 Kidney Transplant Study Group. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. Transplantation. 1997. 63:977–983.
2. Margreiter R. European Tacrolimus vs Ciclosporin Microemulsion Renal Transplantation Study Group. Efficacy and safety of tacrolimus compared with ciclosporin microemulsion in renal transplantation: a randomised multicentre study. Lancet. 2002. 359:741–746.
3. Mehmet H. Caspases find a new place to hide. Nature. 2000. 403:29–30.
4. Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000. 403:98–103.
5. Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002. 277:34287–34294.
6. Jordan ML, Naraghi R, Shapiro R, Smith D, Vivas CA, Scantlebury VP, et al. Tacrolimus rescue therapy for renal allograft rejection--five-year experience. Transplantation. 1997. 63:223–228.
7. Schleibner S, Krauss M, Wagner K, Erhard J, Christiaans M, van Hooff J, et al. FK 506 versus cyclosporin in the prevention of renal allograft rejection--European pilot study: six-week results. Transpl Int. 1995. 8:86–90.
8. Gao Z, Huang K, Xu H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol Res. 2001. 43:173–178.
9. Halliwell B, Gutteridge JMC. Halliwell B, Gutteridge JMC, editors. Protection aganist oxidants in biological systems: the superoxide theory of oxygen toxicity. Free Radicals in Biology and Medicine. 1989. 2nd ed. Oxford: Clarendon Press;86–89.
10. Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968. 61:748–755.
11. Stocker R, McDonagh AF, Glazer AN, Ames BN. Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods Enzymol. 1990. 186:301–309.
12. Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993. 259:381–384.
13. Tanaka S, Akaike T, Fang J, Beppu T, Ogawa M, Tamura F, et al. Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumour. Br J Cancer. 2003. 88:902–909.
14. Kroncke KD, Fehsel K, Kolb-Bachofen V. Nitric oxide: cytotoxicity versus cytoprotection--how, why, when, and where? Nitric Oxide. 1997. 1:107–120.
15. Messmer UK, Brune B. Nitric oxide-induced apoptosis: p53-dependent and p53-independent signalling pathways. Biochem J. 1996. 319(Pt 1):299–305.
16. Gotoh T, Mori M. Nitric oxide and endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol. 2006. 26:1439–1446.
17. Liu YH, Carretero OA, Cingolani OH, Liao TD, Sun Y, Xu J, et al. Role of inducible nitric oxide synthase in cardiac function and remodeling in mice with heart failure due to myocardial infarction. Am J Physiol Heart Circ Physiol. 2005. 289:H2616–H2623.
18. Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005. 115:2656–2664.
19. Zhou J, Lhotak S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005. 111:1814–1821.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download