Abstract
Gastric cancer remains still the most frequent type of cancer despite its declining incidence in Korea. As a result of the health promotion policy of the Korean government and increase in concern for individuals' health, screening endoscopy for detecting early gastric cancer and general physical exams have become widespread. Thereby, the incidental detection of gastric submucosal tumors (SMTs) are now occasionally diagnosed by screening endoscopy. Since endoscopic examination gives little information on SMTs, a variety of studies have been conducted to determine the etiology of SMTs and to distinguish them from extra-luminal compressive lesions. Here, we report one clinical case of cavernous hemangioma on the left lateral section of the liver and one case of omental cyst, which was preoperatively mistaken as gastric SMT.
Since endoscopic biopsy rarely gives information on SMTs, surgical resection is generally preferred for diagnosing and treating potential cases of SMTs. The stomach is surrounded by other organs, such as the liver, gall bladder, spleen, pancreas, and transverse colon. Sufficient inflation of the stomach is mandatory for detail examination during endoscopy, and this causes the stomach to contact adjacent organs. If an abnormal lesion exists, especially a protruding cyst or neoplasm, the compressed region can be mistaken for a gastric submucosal tumor.(1) To avoid this problem, additional studies: endoscopic ultrasonography (EUS), abdominal computed tomography (CT) and magnetic resonance imaging (MRI) may be needed.
A 50-year-old female was referred to our clinic for treatment of a gastric lesion. She had been suffering from epigastric discomfort for 6 months. A protruding mass, 3.5×3.0 cm in size located at the gastric fundus was detected by gastrofiberscope and the contrast study showed similar results (Fig. 1A). EUS results suggested gastric SMT located in the fourth layer of gastric wall (Fig. 1B) and abdominal CT finding suggested gastric hemangioma (Fig. 1C). Therefore, we decided to perform a laparoscopic gastric wedge resection. Upon insertion of the electrolaparoscope, an oval-shaped mass hanging from the left lateral section of the liver was found (Fig. 1D), however, no mass was palpated in the gastric fundus. We then performed intraoperative gastrofiberscope. During the procedure, we could not clearly observe the gastric lesion suspected for gastric hemangioma because the suspected lesion only occasionally appeared in company with inflation of the stomach. We concluded that there was no actual gastric lesion and that the suspected lesion was created by compression of the mass of the liver. We performed laparoscopic mass resection. The patient recovered without complications. The lesion was determined to be a cavernous hemangioma.
A 47-year-old female presented at our clinic with a gastric mass suspected to be a duplicated cyst of the stomach. She complained of intermittent dyspepsia. EUS examination showed this lesion to lie on the posterior wall of the fundus in the stomach, and interpreted it to be a duplication cyst or pancreatic cyst (Fig. 2A). Since patient had frequent abdominal bloating after meal, we performed laparoscopic exploration. Hepatogastric ligament was divided along the lesser curvature of the stomach for exposure of omental busa, and then, with elevation of the stomach, there was a protruding cystic mass, which compresses the stomach and measured about 5×4 cm size (Fig. 2B). After a full survey of the stomach, we concluded that the gastric lesion was an artificial lesion created by a cystic mass positioned on the posterior wall of the stomach. The cyst was resected and diagnosed as an omental cyst.
A SMT has been described as a growth under the mucosa of the gastrointestinal tract whose presence cannot be confirmed by endoscopy or barium radiography.(2) On endoscopic examination, the mucosa appears to be normal, but ulceration of the mucosa is occasionally observed in cases of large SMT. Although most gastric SMTs do not produce any symptoms, gastric SMTs located near the cardia or the pylorus may occasionally produce obstructive symptoms and extrinsic compression of the stomach may also cause dyspepsia, epigastralgia, or tenderness.(1) The symptoms of SMT and extrinsic compression of the stomach vary based on the lesion's size. In general, these symptoms arise more often when SMT size is >3 cm.(1)
Several organs can cause extrinsic compression of the stomach and hence mimic gastric SMT. Under normal anatomical conditions, the spleen and splenic vessel are the most common causes of compression and followed by pancreas and gallbladder.(1,3) The most frequent cause of external compression of the stomach depends on the part of the stomach under consideration: it is the spleen in the case of the fundus and cardia, the liver in the case of the body and anterior wall, the pancreas in the case of the body and posterior wall, and the gall bladder in the case of the antrum.(3) In addition, pathological conditions, such as hepatic hemangioma, distended gallbladder, pancreatic cyst, and splenic artery aneurysm can cause extrinsic compression of the stomach.(1,3)
Since endoscopy and barium radiography are insufficient to determine the etiology of SMT, additional diagnostic methods are necessary. Various diagnostic studies are available; abdominal CT, magnetic resonance imaging (MRI), and endoscopic ultrasonography (EUS) are frequently used.(4) Abdominal CT and MRI are used to delineate the border of an SMT to distinguish it from adjacent structures and to determine its size. However, these methods offer insufficient insight into confirming a diagnosis of SMT.(4) EUS is known as a useful method for differentiating submucosal lesions from extrinsic compression and is recommended as a first choice for examining gastric SMT.(1,2,5) Rösch et al.(1) reported that the sensitivity and specificity of endoscopy for differentiating SMT from lesions created by extrinsic compression were 87% and 29% and the corresponding values for EUS were 92% and 100%, respectively. It has also been reported that lesions created by extrinsic compression normally present five compressed layers, as observed by EUS.(2) In our cases, however, all patients underwent EUS, and the EUS findings did not coincide with operative findings. There was one case of hepatic cyst misdiagnosed as gastric SMT.(6) In this case, endoscopy, abdominal CT, and EUS were preoperatively performed, but the problem was ultimately found to be extrinsic compression of the stomach.
Several observations during endoscopy and EUS may be helpful for distinguishing SMT from extrinsic compression: observing the change in location and appearance of the mass as the position of the patient changes, observing the mass during air inflation or deflation, or observing the change of the mass's shape when touched with biopsy forceps.(3,5,6) Even with these approaches, clearly distinguishing the two phenomena remains difficult.
It is also difficult to determine the characteristics of SMT because endoscopic biopsy nearly always contains mucosa. With advances in laparoscopic surgery, laparoscopic resection for cavernous hemangioma of the liver and omental cyst has become feasible and is performed regularly by surgeons.(7-9) In our cases, cavernous hepatic hemangioma was in the left lateral section and was easily resected by electrocautery and endoscopic stapler. Another omental cyst was excised by electrocautery and harmonic scalpel (Ethicon-Surgery, Cincinnati, OH, USA).
In conclusion, extra-gastric lesions may mimic gastric SMT. Especially, if they have cystic nature in EUS, the surgeon should keep in mind the possibility of extrinsic gastric compression of cystic lesions. Although EUS is the most reliable diagnostic modality for SMT, it is not infallible. Therefore, suspected SMTs in the cardia and fundus of the stomach should be carefully evaluated, and if warranted, laparoscopic resection is a feasible and safe procedure.
Figures and Tables
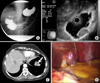 | Fig. 1Barium radiography of stomach shows protruding mass in gastric fundus (A), EUS shows hypoechoic homogenous mass in 4th layer of the gastric wall (B), Abdominal CT shows gastric mass in fundus and arrow indicates hemangioma (C), Cavernous hepatic hemangioma in left lateral section of the liver (D). |
References
1. Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, et al. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002. 37:856–862.
2. Chak A. EUS in submucosal tumors. Gastrointest Endosc. 2002. 56:S43–S48.
3. Chen TK, Wu CH, Lee CL, Lai YC, Yang SS, Tu TC. Endoscopic ultrasonography to study the causes of extragastric compression mimicking gastric submucosal tumor. J Formos Med Assoc. 2001. 100:758–761.
4. Ponsaing LG, Kiss K, Loft A, Jensen LI, Hansen MB. Diagnostic procedures for submucosal tumors in the gastrointestinal tract. World J Gastroenterol. 2007. 13:3301–3310.
5. Motoo Y, Okai T, Ohta H, Satomura Y, Watanabe H, Yamakawa O, et al. Endoscopic ultrasonography in the diagnosis of extraluminal compressions mimicking gastric submucosal tumors. Endoscopy. 1994. 26:239–242.
6. Park JM, Kim J, Kim HI, Kim CS. Hepatic cyst misdiagnosed as a gastric submucosal tumor: a case report. World J Gastroenterol. 2008. 14:3092–3094.
7. Lerner SM, Hiatt JR, Salamandra J, Chen PW, Farmer DG, Ghobrial RM, et al. Giant cavernous liver hemangiomas: effect of operative approach on outcome. Arch Surg. 2004. 139:818–821.
8. Patriti A, Graziosi L, Sanna A, Gullà N, Donini A. Laparoscopic treatment of liver hemangioma. Surg Laparosc Endosc Percutan Tech. 2005. 15:359–362.
9. Conzo G, Vacca R, Grazia Esposito M, Brancaccio U, Celsi S, Livrea A. Laparoscopic treatment of an omental cyst: a case report and review of the literature. Surg Laparosc Endosc Percutan Tech. 2005. 15:33–35.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download