Abstract
Purpose
Long-term exposure to extremely low-frequency (60 Hz) electromagnetic fields (ELF-EMF) raises the questions of the induction of biological effects including tumorigenesis. One mechanism through which ELF-MFS could influence neoplastic development is the imbalance of cellular proliferation and cell apoptosis. The present study investigated the effect of ELF-EMF on chemically-induced thyroid carcinogenesis in a rat.
Methods
We examined cellular proliferation index measured by anti-Ki-67 antigen, apoptosis, apoptosis related proteins such as caspase 3 and p53, and cell cycle-related proteins (cyclin D1 and p21WAF1/Cip1). Forty Male F344 rats received a subcutaneous N-bis(2-hydroxypropyl)nitrosamine (DHPN, 2,800 mg/kg) injection, and 1 week later were allowed free access to drinking water containing sulfadimethoxine (0.1%) for 12 weeks. Twenty rats were exposed by ELF-EMF. During the carcinogenesis, sequential histological changes from hyperplasia, adenoma, and ultimately to overt carcinomas were noted.
Results
The exposure group of ELF-EMF, significantly increases the number size of carcinomas. Also, the proliferative and apoptotic indices were significantly increased in the ELF-EMF exposure group than in the control group. The caspase 3 protein expression did not show any significant changes between ELF-EMF group and control group. The p53 protein was not detected in both ELF-EMF exposure and control group. Among the cell cycle related proteins, cyclin D1, not p21WAF1/Cip1, was significantly increased in adenomas and carcinomas in ELF-EMF exposure group compared with the control group.
Figures and Tables
Fig. 2
N-bis(2-hydroxypropyl)nitrosamine and sulfadimethoxine induced rat thyroid lesions, showing follicular cell hyperplasia (A), adenoma (B), and carcinoma (C) (H&E, ×200).
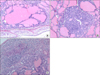
Fig. 3
Immunohistochemical reactivity of Ki-67 (A), p21WAF1/Cip1 (B), cyclin D1 (C), and caspase 3 (D) expression (ABC, ×200).
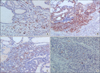
Table 1
Thyroid lesions in rat after 12 weeks treated with N-bis (hydroxypropyl)nitrosamine and sulfadimethoxine

References
1. Adey WR. Tissue interactions with nonionizing electromagnetic fields. Physiol Rev. 1981. 61:435–514.
2. McCann J, Dietrich F, Rafferty C. The genotoxic potential of electric and magnetic fields: an update. Mutat Res. 1998. 411:45–86.
3. Minder CE, Pfluger DH. Leukemia, brain tumors, and exposure to extremely low frequency electromagnetic fields in Swiss railway employees. Am J Epidemiol. 2001. 153:825–835.
4. Cho YH, Chung HW. The effect of extremely low frequency electromagnetic fields (ELF-EMF) on the frequency of micronuclei and sister chromatid exchange in human lymphocytes induced by benzo(a)pyrene. Toxicol Lett. 2003. 143:37–44.
5. Imai T, Onose J, Hasumura M, Ueda M, Takizawa T, Hirose M. Sequential analysis of development of invasive thyroid follicular cell carcinomas in inflamed capsular regions of rats treated with sulfadimethoxine after N-bis(2-hydroxypropyl)nitrosamine-initiation. Toxicol Pathol. 2004. 32:229–236.
6. Shimo T, Mitsumori K, Onodera H, Takahashi M, Ueno Y, Katayama J, et al. Effect of rat thyroid proliferative lesion development by intermittent treatment with sulfadimethoxine. Cancer Lett. 1995. 96:209–218.
7. Boorman GA, Eustis SL, Elwell MR, Montgomerry CA, Mackenzie WF. Pathology of the Fischer Rat. 1990. 1st ed. San Diego: Academic Press;524–539.
8. Pitot HC, Sirica AE. The stages of initiation and promotion in hepatocarcinogenesis. Biochim Biophys Acta. 1980. 605:191–215.
9. National Institute of Environmental Health Sciences (NIEHS). NIH publication No. 98-3981. Assessment of Health Effects from Exposure to Power-line Frequency Electric and Magnetic Fields: Working Group Report. Research Triangle Park, NC:
10. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Non-Ionizing Radiation, Part 1: Static and Extremely Low-Frequency (ELF) Electric and Magnetic Fields. 2002. Lyon, France: IARC Press;429.
11. Gobba F, Malagoli D, Ottaviani E. Effects of 50 Hz magnetic fields on fMLP-induced shape changes in invertebrate immunocytes: The role of calcium ion channels. Bioelectromagnetics. 2003. 24:277–282.
12. Stuchly MA, McLean JR, Burnett R, Goddard M, Lecuyer DW, Mitchel RE. Modification of tumor promotion in the mouse skin by exposure to an alternating magnetic field. Cancer Lett. 1992. 65:1–7.
13. Yokus B, Cakir DU, Akdag MZ, Sert C, Mete N. Oxidative DNA damage in rats exposed to extremely low frequency electro magnetic fields. Free Radic Res. 2005. 39:317–323.
14. Brown DC, Gatter KC. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990. 17:489–503.
15. Wolf FI, Torsello A, Tedesco B, Fasanella S, Boninsegna A, D'Ascenzo M, et al. 50-Hz extremely low frequency electromagnetic fields enhance cell proliferation and DNA damage: possible involvement of a redox mechanism. Biochim Biophys Acta. 2005. 1743:120–129.
16. Tofani S, Cintorino M, Barone D, Berardelli M, De Santi MM, Ferrara A, et al. Increased mouse survival, tumor growth inhibition and decreased immunoreactive p53 after exposure to magnetic fields. Bioelectromagnetics. 2002. 23:230–238.
17. Loberg LI, Engdahl WR, Gauger JR, McCormick DL. Cell viability and growth in a battery of human breast cancer cell lines exposed to 60 Hz magnetic fields. Radiat Res. 2000. 153:725–728.
18. Biscotti CV, Hart WR. Apoptotic bodies: a consistent morphologic feature of endocervical adenocarcinoma in situ. Am J Surg Pathol. 1998. 22:434–439.
19. Lee JS, Ahn SS, Jung KC, Kim YW, Lee SK. Effects of 60 Hz electromagnetic field exposure on testicular germ cell apoptosis in mice. Asian J Androl. 2004. 6:29–34.
20. Sheikh MS, Huang Y. TRAIL death receptors, Bcl-2 protein family, and endoplasmic reticulum calcium pool. Vitam Horm. 2004. 67:169–188.
21. Ding GR, Nakahara T, Tian FR, Guo Y, Miyakoshi J. Transient suppression of X-ray-induced apoptosis by exposure to power frequency magnetic fields in MCF-7 cells. Biochem Biophys Res Commun. 2001. 286:953–957.
22. Harland J, Engstrom S, Liburdy R. Evidence for a slow time-scale of interaction for magnetic fields inhibiting tamoxifen's antiproliferative action in human breast cancer cells. Cell Biochem Biophys. 1999. 31:295–306.
23. Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997. 275:1132–1136.
24. Goto A, Sakamoto A, Machinami R. An immunohistochemical analysis of cyclin D1, p53, and p21waf1/cip1 proteins in tumors originating from the follicular epithelium of the thyroid gland. Pathol Res Pract. 2001. 197:217–222.
25. Zou M, Shi Y, Farid NR, al-Sedairy ST. Inverse association between cyclin D1 overexpression and retinoblastoma gene mutation in thyroid carcinomas. Endocrine. 1998. 8:61–64.
26. Lange S, Viergutz T, Simko M. Modifications in cell cycle kinetics and in expression of G1 phase-regulating proteins in human amniotic cells after exposure to electromagnetic fields and ionizing radiation. Cell Prolif. 2004. 37:337–349.
27. Zhang H, Xiong Y, Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol Biol Cell. 1993. 4:897–906.
28. Ito Y, Kobayashi T, Takeda T, Komoike Y, Wakasugi E, Tamaki Y, et al. Expression of p21 (WAF1/CIP1) protein in clinical thyroid tissues. Br J Cancer. 1996. 74:1269–1274.
29. Lange S, Richard D, Viergutz T, Kriehuber R, Weiss DG, Simko M. Alterations in the cell cycle and in the protein level of cyclin D1, p21CIP1, and p16INK4a after exposure to 50 Hz MF in human cells. Radiat Environ Biophys. 2002. 41:131–137.
30. Czyz J, Nikolova T, Schuderer J, Kuster N, Wobus AM. Non-thermal effects of power-line magnetic fields (50 Hz) on gene expression levels of pluripotent embryonic stem cells-the role of tumour suppressor p53. Mutat Res. 2004. 557:63–74.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download