Abstract
Diffuse large B cell lymphoma is the most common type of non-Hodgkin's lymphoma, representing approximately one-third of all cases and involving the gastrointestinal tract in about 18%. With the development of modern chemotherapeutic regimens and advances in medical care, the prognosis for malignant lymphoma can be excellent. However, because of the aggressive adjuvant therapy required, complications such as bowel perforation may be fatal. In cases of chemotherapy for malignant lymphoma, we should keep in mind the possibility of perforation of the bowel after chemotherapy. Early detection is important to save patients.
In diffuse large B cell lymphoma, combination chemotherapy is the initial treatment and CHOP is the treatment of choice.(1) Using this approach, 60 to 70% of patients are expected to achieve complete remission, 50 to 70% of complete responders will be cured, and the 5-year survival is 46%.(2) Fu and Perzin(3) reported that the incidence of perforation in small intestinal malignant lymphoma is about 10%. Sakakura et al.(4) also described one case of bowel perforation during chemotherapy with no lymphoma cells seen on pathological examination. In addition, Libicher et al.(5) reported cicatricial jejunal stenosis after chemotherapy for gastrointestinal lymphoma; their patient underwent surgical resection, and they found no histological evidence of lymphoma recurrence.
We report our experience of three cases of bowel perforation, in which no lymphoma cells were found, after chemotherapy for diffuse large B cell lymphoma.
A 29-year-old female was hospitalized via our emergency room with diffuse abdominal tenderness. She was diagnosed, via histology, with diffuse large B cell lymphoma of the tonsil (Fig. 1A) in August 2003, and received a second cycle of CHOP + rituximab combination chemotherapy 45 days prior to visiting.
Her peripheral laboratory data showed WBC 3,200/mm3, Hb 13.1 g/dl, platelet count 289,000/mm3, and amylase 195 U/L. Others values were within the normal range. Abdominal computed tomography detected hemoperitoneum and pneumoperitoneum with diffuse peritonitis (Fig. 2A).
We carried out emergency surgery that day. We found a perforation of the small bowel with no recurrent mass. The operation consisted of small bowel segmental resection and anastomosis. There was mild mucosal destruction with an irregular surface, intense inflammatory cell infiltration, and perforation without lymphoma cells in the pathological specimen examined postoperatively (Fig. 3A).
The second patient was a 61-year-old male who visited the emergency room because of right lower quadrant pain. He had been diagnosed with terminal ileal lymphoma (diffuse large B cell lymphoma) (Fig. 1B) and underwent a right hemicolectomy on 8 October 2003. He received a second course of CHOP + rituximab chemotherapy 39 days prior to visiting.
The peripheral blood laboratory data showed WBC 16,600/mm3, Hb 9.2 g/dl, platelet count 236,000/mm3, and albumin 2.9 g/dl; the other values were normal. Abdominal computed tomography showed high amounts of free air in the peritoneal cavity (Fig. 2B).
We performed emergency surgery and found a perforation in the colon in the distal portion of the previous anastomotic site. Segmental resection of the perforation site and anastomosis was performed.
The bowel mucosa showed ischemic changes, submucosal edema, hemorrhage, and serosal inflammation with no lymphoma cells in the histology (Fig. 3B).
A 12-year-old girl had lower abdominal pain and consulted the Department of Pediatrics. She received NHL-BMF 90 chemotherapy for a diffuse large B cell lymphoma in the lower abdomen (Fig. 1C) 63 days previous to this visit.
The peripheral blood laboratory findings showed WBC 34,700/mm3, Hb 10.8 g/dl, platelet count 175,000/mm3, and LDH 556 U/L; everything else was normal. Computed tomography strongly suggested a bowel perforation (Fig. 2C).
She consulted us on 5 December 2003, and an emergency operation was performed the next day. An ileal perforation was present, and we carried out a small bowel segmental resection and anastomosis.
The postoperative pathological examination showed many foamy macrophages and multinucleated giant cells in the submucosa and muscle proper, serosal reaction, and a focal segment of mucosal ulceration with no lymphoma cells (Fig. 3C).
The three patients in our series developed bowel perforations 5 to 9 weeks after their first chemotherapy session for diffuse large B cell lymphoma, and the primary site of the tumor differed in each case (tonsil, terminal ileum, and intra-abdominal mass). No lymphoma cells were present in the postoperative specimens. All the patients survived and have continued chemotherapy for lymphoma 3 to 8 weeks after surgery.
Perforation of the gastrointestinal tract whether tumor related or chemotherapeutically induced is a serious complication and may be fatal. Bowel perforation in patients with primary malignant lymphoma usually occurs at the site of tumor. We report our experience of three cases of bowel perforation, in which no lymphoma cells were found, after chemotherapy for diffuse large B cell.
Lundy et al.(6) reported 36 carcinoma patients with spontaneous intestinal perforation; 21 of these perforations were caused by tumor necrosis, and eight patients were receiving chemotherapy or corticosteroids at the time of perforation. Only 19 of 36 patients were explored, with an operative mortality of 68%. Meyers et al.(7) described six cases of intestinal perforation during induction chemotherapy for non-Hodgkin's lymphoma. Sherlock and Oropezar(8) also reported a case who developed multiple intestinal perforations 1 week after alkylating agent therapy for lymphoma. Jones and Abramson(9) found a strong relationship between the use of cytosine arabinoside and subsequent perforation in 14 patients who underwent induction therapy for leukemia. The exact etiology is unknown and the mechanism can be complex. A toxic effect of chemotherapeutic agents on rapidly dividing tissues, such as gastrointestinal mucosa, that causes epithelial ulceration is the most probable etiology.(10)
Beck et al.(11) first reported an association between corticosteroid therapy and gastrointestinal perforation in 1950. Glenn and Grafe(12) suggested that corticosteroids inhibit mucosal cell and fibroblast proliferation, impairing normal reparative activity in the bowel wall, and that the bowel is perforated as a result. Furthermore, corticosteroid therapy often mutes the signs and symptoms of peritonitis, delaying diagnosis and treatment. The reported mortality rates for intestinal perforation in patients receiving corticosteroids range from 27 to 100%.(13,14)
Commonly, gastrointestinal perforation occurs at sites involving lymphoma cells. Nevertheless, the bowel can also be perforated during chemotherapy or corticosteroid therapy with no lymphoma cell invasion, as in our cases.(7) Bowel perforation is a devastating complication of non-Hodgkin's lymphoma. Therefore, physicians and surgeons treating non-Hodgkin's lymphoma should be alert to its possible occurrence and should perform prompt and aggressive diagnosis and treatment. Therefore, early diagnosis and treatment are important to save the patient, because the mortality rate is very high.
Figures and Tables
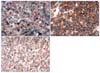 | Fig. 1Immunohistochemical staining for CD 79. There were large nuclei in each case. Tonsil (A), Terminal ileum (B), Intra-abdominal mass (C)(Immunohistochemical staining, ×400). |
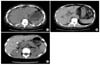 | Fig. 2Abdominal computed tomography. A large amount of fluid and free air (arrow) in the peritoneal cavity (A). Multiple pockets of free air were present in the peritoneal cavity and Morrison's pouch (B). A small area of free air was present around the porta hepatis (C). |
 | Fig. 3The area of perforated bowel. Mild mucosal destruction with an irregular surface, intense inflammatory cell infiltration, and perforation (A). The bowel showed mucosal ischemic changes and submucosal edema, with hemorrhage and serosal inflammation (B). The specimen contained many foamy macrophages and multinucleated giant cells in the submucosa and muscle proper, with serosal reaction and a focal segment of mucosal ulceration (C)(H&E, ×20). |
References
1. Hiddemann W. Non-Hodgkin's lymphomas--current status of therapy and future perspectives. Eur J Cancer. 1995. 31:2141–2145.
2. James OA, Dan LL. Malignancies of Lymphoid Cells. Harrison's Principles of Internal Medicine. 2001. 15th ed. New York: McGraw-Hill;715–727.
3. Fu YS, Perzin KH. Lymphosarcoma of the small intestine. A clinicopathologic study. Cancer. 1972. 29:645–659.
4. Sakakura C, Hagiwara A, Nakanishi M, Yasuoka R, Shirasu M, Togawa T, et al. Bowel perforation during chemotherapy for non-hodgkin's lymphoma. Hepatogastroenterology. 1999. 46:3175–3177.
5. Libicher M, Lamade W, Kasperk C, Grenacher L, Kauffmann GW. Cicatricial small intestinal stenosis following chemotherapy for a gastrointestinal lymphoma. Dtsch Med Wochenschr. 1996. 121:1359–1362.
6. Lundy J, Sherlock P, Kurtz R, Fortner JG, Turnbull AD. Spontaneous perforation of the gastrointestinal tract in patients with cancer. Am J Gastroenterol. 1975. 63:447–450.
7. Meyers PA, Potter VP, Wollner N, Exelby P. Bowel perforation during initial treatment for childhood non-Hodgkin's lymphoma. Cancer. 1985. 56:259–261.
8. Sherlock P, Oropezar . Jejunal perforations in lymphoma after chemotherapy. Arch Intern Med. 1962. 110:102–107.
9. Jones GT, Abramson N. Gastrointestinal necrosis in acute leukemia: a complication of induction therapy. Cancer Invest. 1983. 1:315–320.
10. Yuen JS, Chow PK, Ahmed Q. Metastatic lung cancer causing bowel perforations: spontaneous or chemotherapy-related? ANZ J Surg. 2002. 72:245–246.
11. Beck JC, Browne JS, Johnson LG. Occurrence of peritonitis during ACTH administration. Can Med Assoc J. 1950. 62:423–426.
12. Glenn F, Grafe WR Jr. Surgical complications of adrenal steroid therapy. Ann Surg. 1967. 65:1023–1032.
13. Rigotti P, Van Buren CT, Payne WD, Peters C, Kahan BD. Gastrointestinal perforations in renal transplant recipients immunosuppressed with cyclosporin. World J Surg. 1986. 10:137–141.
14. Greenstein AJ, Sachar DB, Mann D, Lachman P, Heimann T, Aufses AH Jr. Spontaneous free perforation and perforated abscess in 30 patients with Crohn's disease. Ann Surg. 1987. 205:72–76.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download