Abstract
Purpose
It is known that DNA methylation is associated with histone acetylation status in regulation of gene expression. In this study, we investigate the effect of demethylating agents and histone deacetylase (HDAC) inhibitor on the tumor suppression and the combined effect of two agents according to methylation status in human colon and breast cancer cell lines.
Methods
In this study, the RKO colorectal cancer cell line, MCF-7 breast cancer cell lines were considered. For each cell line, we used HDAC inhibitor sodium butyrate (SB), demethylating agent 5-aza-2'-deoxycytidine (5-aza-DC) and a combination of both agents. We estimated the percentage of cell survival using the XTT method and experimented with the augmentative effects of both agents.
Results
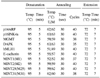
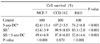
In RKO cell line in which most of the genes are methylated, 74% of cell survival was shown for 5-aza-DC treatment and 83% of cell survival for SB treatment. In MCF-7 cell line that approximately half of the genes are methylated, 82% cell survival was shown for 5-aza-DC treatment and 63% cell survival for SB treatment. We observed that the survival fraction is lower after the combined treatment of 5-aza-DC and SB than that of 5-aza-DC or SB alone in both RKO (53%) and MCF-7 (49%) cell lines (P<0.001).
Conclusion
For highly methylated genes, 5-aza-DC is more effective on the tumor suppression than SB. On the other hand, if the methylation of the promoter region is at low density, SB is noted to be more effective than 5-aza-DC. Furthermore, the combined treatment of 5-aza-DC and SB is more effective than using each agent alone.
Figures and Tables
 | Fig. 1MS-PCR after 5-aza-2'-deoxycytidine (5-aza-DC) treatment. In the control group, most of the genes were methylated, but cell lines treated with aza-DC showed that demethylated bands and methylated bands were slightly decreased. |
 | Fig. 2MS-PCR after sodium butyrate treatment. Compared to the control group, there were almost no changes in methylation status with the addition of sodium butyrate. |
References
1. Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002. 3:415–428.
2. Szyf M, Pakneshan P, Rabbani SA. DNA methylation and breast cancer. Biochem Pharmacol. 2004. 68:1187–1197.
3. Garinis GA, Patrinos GP, Spanakis NE, Menounos PG. DNA hypermethylation: when tumour suppressor genes go silent. Hum Genet. 2002. 111:115–127.
4. Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002. 108:475–487.
5. Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001. 1:194–202.
6. Verma M, Srivastava S. Epigenetics in cancer: implications for early detection and prevention. Lancet Oncol. 2002. 3:755–763.
7. Walton TJ, Li G, Seth R, McArdle SE, Bishop MC, Rees RC. DNA demethylation and histone deacetylation inhibition cooperate to re-express estrogen receptor beta and induce apoptosis in prostate cancer cell-lines. Prostate. 2008. 68:210–222.
8. Murakami J, Asaumi J, Maki Y, Tsujigiwa H, Kuroda M, Nagai N, et al. Effects of demethylating agent 5-aza-2(')-deoxycytidine and histone deacetylase inhibitor FR901228 on maspin gene expression in oral cancer cell lines. Oral Oncol. 2004. 40:597–603.
9. Bar-Sela G, Jacobs KM, Gius D. Histone deacetylase inhibitor and demethylating agent chromatin compaction and the radiation response by cancer cells. Cancer J. 2007. 13:65–69.
10. Zhu WG, Otterson GA. The interaction of histone deacetylase inhibitors and DNA methyltransferase inhibitors in the treatment of human cancer cells. Curr Med Chem Anticancer Agents. 2003. 3:187–199.
11. Shen WJ, Dai DQ, Teng Y, Liu HB. Regulation of demethylation and re-expression of RASSF1A gene in gastric cancer cell lines by combined treatment of 5-Aza-CdR and NaB. World J Gastroenterol. 2008. 14:595–600.
12. Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002. 1:287–299.
13. Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000. 92:1210–1216.
14. Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999. 21:103–107.
15. Zhu WG, Lakshmanan RR, Beal MD, Otterson GA. DNA methyltransferase inhibition enhances apoptosis induced by histone deacetylase inhibitors. Cancer Res. 2001. 61:1327–1333.
16. Fuks F, Burgers WA, Godin N, Kasai M, Kouzarides T. Dnmt3a binds deacetylases and is recruited by a sequence-specific repressor to silence transcription. EMBO J. 2001. 20:2536–2544.
17. Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998. 393:386–389.
18. Kondo Y, Issa JP. Epigenetic changes in colorectal cancer. Cancer Metastasis Rev. 2004. 23:29–39.
19. Zhu WG, Hileman T, Ke Y, Wang P, Lu S, Duan W, et al. 5-aza-2'-deoxycytidine activates the p53/p21Waf1/Cip1 pathway to inhibit cell proliferation. J Biol Chem. 2004. 279:15161–15166.
20. Wang H, Zhao Y, Li L, McNutt MA, Wu L, Lu S, et al. An ATM- and Rad3-related (ATR) signaling pathway and a phosphorylation-acetylation cascade are involved in activation of p53/p21Waf1/Cip1 in response to 5-aza-2'-deoxycytidine treatment. J Biol Chem. 2008. 283:2564–2574.
21. Chai G, Li L, Zhou W, Wu L, Zhao Y, Wang D, et al. HDAC inhibitors act with 5-aza-2'-deoxycytidine to inhibit cell proliferation by suppressing removal of incorporated abases in lung cancer cells. PLoS ONE. 2008. 3:e2445.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download