Abstract
Although prosthetic materials are commonly used to repair abdominal wall defects, they are also associated with postoperative complications. These complications could be prevented by the adoption of uniform guidelines on surgical methods and materials, but the best anatomical position for placement of prosthetic meshes is unclear. We report a case of an enterocutaneous fistula that developed after an abdominal wall defect was repaired by intraperitoneal application of a prosthetic mesh (Marlex®) to raise awareness of the consequences of improper use of prosthetic materials.
Although surgical techniques for repairing abdominal wall defects have improved in recent years, recurrence is still common. Prosthetic mesh is widely used for repairing abdominal wall defects, but there are no established methods or guidelines for reducing postoperative complications associated with this technique. The efficacy of intraperitoneal mesh for abdominal wall defects is controversial. Some studies have shown that intraperitoneal polypropylene mesh with omental coverage is effective and results in few complications, but others have indicated that polypropylene meshes are associated with a high incidence of postoperative complications when they are used within the peritoneum. Therefore, intraperitoneal placement of mesh should be avoided whenever possible. In this report, we describe a case of intestinal fistula that developed after an abdominal wall defect was repaired by peritoneal placement of high-density polypropylene mesh (Marlex®).
A 57-year-old female patient presented with painful bleeding and leakage of fecal material from the site of a previous abdominal incision, which was located to the right of the umbilicus. She underwent a left hemicolectomy and a colostomy at a provincial hospital because of colon cancer 15 years previously. She also underwent a subtotal colectomy and an ileostomy because of colon infarction induced by a mesenteric thromboembolism after a traffic accident 3 years after the first operation. Extensive debridement was performed because necrotizing fasciitis of the abdominal wall and evisceration developed 5 days after the operation. Seven days after the operation, abdominal wall reconstruction using intraperitoneal placement of high-density polypropylene mesh (Marlex®) was performed without placing tension on the abdominal wall.
Five years after the second operation, the patient presented at the Department of General Surgery of our hospital complaining of fecal discharge from a wound that had formed around the umbilicus as a result of the previous operation. Physical examination revealed that the fecal material was passing through a fistula which had developed 3 cm above the umbilicus at the site of previous midline incision. The patient declined surgical intervention because of cachexia and the adhesive state of the infected wound. The enterocutaneous fistula was managed conservatively using periodic dressing and medication. After 7 years of conservative treatment, the length of the fistula increased to 7 cm and the patient acceded to operative treatment.
Computed tomography revealed a tubular structure, which appeared to be a part of the bowel, protruding through the defect on the midline between the rectus abdominis muscles. The structure and adjacent parietal peritoneum exhibited diffuse wall thickening and prominent enhancement (Fig. 1A). A fistulogram revealed a long ileocutaneous fistula, which was coincident with the clinical findings (Fig. 1B).
A 15 cm long midline abdominal incision was made under general anesthesia. Three pieces of polypropylene mesh from the previous operation were discovered during sharp dissection of the mucosa of the fistula. The mesh adhered to the tissue and had infiltrated abdominal wall tissue in the proximity of the tract of the fistula. The pieces of mesh were removed by sharp dissection. The pieces were gray to tan in color, and the associated soft tissue had an irregular appearance. The largest piece was 7.8×4.5 cm (Fig. 2). The mucosa of the fistula tract was dissected away from the surrounding adherent tissue. The rim of the perforated layer of mucosa was debrided and closed with a double layer of tissue. Finally, the abdominal layers were closed using interrupted one-layer non-absorbable sutures. Two silastic drains were inserted into the subcutaneous tissue.
Pathological examination showed that the mesh had induced an immune reaction that resulted in accumulation of inflamed granular tissue and hemorrhagic, necrotizing inflammation (Fig. 3). There was no evidence of fecal material from the wound at the 6-month follow-up visit.
The annual number of operations performed in Korea to repair abdominal wall defects, including hernias, is in excess of 30,000, which is 70% more than that performed 5 years ago. However, the introduction of tension-free surgical techniques and prosthetic material has reduced the incidence of recurrence from 20~30% to 0~10%.(1)
Serious complications are mainly associated with the position of prosthetic meshes relative to the abdominal wall. Mesh may be positioned in three ways: onlay, sublay (preperitoneal), and intraperitoneal.(2) The intraperitoneal position is commonly used in combination with omental interposition, which is applied to close the peritoneum without applying tension. Ideally, the prosthesis would become tightly incorporated into the abdominal wall by scar tissue formed during the regeneration process. In this position, the prosthetic mesh is in contact with the bowel, which may cause dense adhesions, mesh migration, mesh erosion into adjacent anatomical structures, and enterocutaneous fistulas. Such complications may promote infection and inhibit movement of the abdominal wall.(2,3)
Infection is the most devastating complication associated with implantation of prosthetic materials.(4) The incidence of infection after primary abdominal wall reconstruction with mesh is 1~2%.(5) LeBlanc(6) concluded that wound infection is a risk factor for hernia recurrence. Therefore, nonabsorbable mesh, which is frequently used to repair large, complex abdominal wall defects, is associated with an increased risk of wound-related complications. Basoglu et al.(7) mentioned that contact between mesh and the bowel should be avoided to prevent complications such as enterocutaneous fistulas.
Vrijland et al.(8) suggested that intraperitoneal placement of polypropylene mesh is safe provided that surgery is conducted under antibiotic cover. Bulic et al.(9) reported that a large, infected abdominal wall defect combined with evisceration and a colostomy because of a gunshot wound was successfully treated with polypropylene mesh reinforcement and free latissimus dorsi muscle-flap coverage, and that the patient's condition 12 months after surgery was good.
These inconsistent results and the multiple options available for managing complex abdominal wall defects may lead to confusion among surgeons. Grevious et al.(10) stated that a thorough understanding of the anatomical structure and function of the abdominal wall is necessary to determine the most appropriate technique for a specific reconstruction. To date, surgery for complex abdominal wall defects has only been successful when knowledge of structural anatomy has been integrated with an understanding of the abdominal wall function of individual patients.
We believe that the risk of developing an enterocutaneous fistula after intraperitoneal placement of Marlex mesh outweighs the benefits of this procedure. When there is direct contact between Marlex mesh and the intestines, mesh erosion and fistula formation are inevitable. Other materials, such as polytetrafluoroethylene (Gore-Tex™, PTFE) mesh, can be substituted for Marlex mesh to prevent the formation of enterocutaneous fistulas after repairing large abdominal wall defects.
The purpose of this report is to raise awareness that prosthetic materials can have adverse consequences. If treatment options for abdominal wall defects are considered on a case-by-case basis, the serious postoperative complications may be prevented.
Figures and Tables
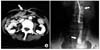 | Fig. 1(A) Abdominal spiral computed tomography (CT). This study revealed tubular structure, which was suspiciously thought to be part of the bowel, was protruded through the defect on the midline between rectus abdominis muscles. This suspicious bowel and adjacent parietal peritoneum showed diffused wall thickening and prominent enhancement. (B) Enterocutaneous fistulogram with Gastrografin for the confirmation of fistula between abdominal skin and ileum. Contrast material was identified at small bowel, but there was no extravasation or collection of contrast material through the fistula tract. |
 | Fig. 2(A) Intraoperative findings of laparotomy for previously indwelt intraperitoneal prosthetic mesh removal. (B) Removed polypropylene prosthetic meshes. These showed grayish, tan, and irregular soft tissue appearances. The larger removed mesh measures 7.8×4.5 cm and the others measure similarly. |
References
1. Leber GE, Garb JL, Alexander AI, Reed WP. Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg. 1998. 133:378–382.
2. Halm JA, de Wall LL, Steyerberg EW, Jeekel J, Lange JF. Intraperitoneal polypropylene mesh hernia repair complicates subsequent abdominal surgery. World J Surg. 2007. 31:423–429.
3. Losanoff JE, Richman BW, Jones JW. Entero-colocutaneous fistula: a late consequence of polypropylene mesh abdominal wall repair: case report and review of the literature. Hernia. 2002. 6:144–147.
4. Engelsman AF, van der Mei HC, Ploeg RJ, Busscher HJ. The phenomenon of infection with abdominal wall reconstruction. Biomaterials. 2007. 28:2314–2327.
5. Deysine M. Pathophysiology, prevention, and management of prosthetic infections in hernia surgery. Surg Clin North Am. 1998. 78:1105–1115.
6. LeBlanc KA. Laparoscopic incisional and ventral hernia repair: complications-how to avoid and handle. Hernia. 2004. 8:323–331.
7. Basoglu M, Yildirgan MI, Yilmaz I, Balik A, Celebi F, Atamanalp SS, et al. Late complications of incisional hernias following prosthetic mesh repair. Acta Chir Belg. 2004. 104:425–428.
8. Vrijland WW, Jeekel J, Steyerberg EW, DenHoed PT, Bonjer HJ. Intraperitoneal polypropylene mesh repair of incisional hernia is not associated with enterocutaneous fistula. Br J Surg. 2000. 87:348–352.
9. Bulic K, Dzepina I, Mijatovic D, Unusic J. Prosthetic mesh for infected abdominal wall defects? Report of a patient with a large full thickness abdominal wall defect and colostomy due to a gunshot wound. J Plast Reconstr Aesthet Surg. 2008. 61:455–458.
10. Grevious MA, Cohen M, Shah SR, Rodriguez P. Structural and functional anatomy of the abdominal wall. Clin Plast Surg. 2006. 33:169–179.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download