Abstract
Purpose
We evaluated differences in the meibographic findings and immunohistochemical structures of mice, rats, and rabbits (commonly used in animal experiments).
Methods
Meibomian gland images were taken using an animal meibograph in mice, rats, and black and white rabbits (five of each animal).The size ratios of the upper and lower Meibomian glands were calculated. Afterm meibographic imaging, the eyes were cut sagittally or transversely and the histological structures were compared using hematoxylin-and-eosin (H&E) and immunohistochemical staining. In addition, the diameters of the central ducts and the areas of ducted glands and the acini were compared using image analysis software.
Results
The meibographic findings were clearer in black rabbits than in the other animals. The upper to lower length ratio of the gland did not differ significantly among the three species. Histologically, no central duct epithelial cells were apparent in mice. The diameters of the central ducts and the areas of ducted glands and acini increased in the following order: mouse, rat, and rabbit. However, the Meibomian gland areas did not differ significantly between mice and rats. On immunohistochemical staining, the central ductal epithelium and the Meibomian gland acini were stained for cytokeratin 5 (CK 5) but only the central ductal epithelium was stained for cytokeratin 6 (CK 6).
Conclusions
The larger the experimental animal, the greater the sizes of the Meibomian gland ducts and acini. In addition, black rabbits yielded better gland images because contrast was enhanced. Compared with sagittal sections of Meibomian glands, transverse sections facilitated a better understanding of the structures of the central ducts and surrounding Meibomian gland acini.
Figures and Tables
 | Figure 1Meibography for animals. (A) Meibography for animals. (B) The animals were anesthetized by intramuscular injection, and meibography of animals was performed. |
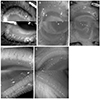 | Figure 2Meibographic images of upper and lower eyelids using Meiboviewer for animal experiments. Black mice (A), white mice (B), white rat (C), black rabbit (D), and white rabbit (E). |
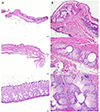 | Figure 3Histologic results of meibomian gland. The structures such as the central duct and the surrounding lobules of the rabbit were observed more uniformly than the other animals. In rats and rabbits, ductal epithelial cells were observed, but in the mouse, epithelial cells of the central duct were not clearly observed (Upper: mouse, Middle: rat, Lower: rabbit) (Hematoxylin and Eosin [H&E] stain, A: ×40, B: ×200). |
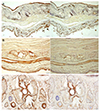 | Figure 4Immunohistochemistry results of meibomian gland (CK 5, CK 6). In the case of mouse, the size of the lobules and the central ducts was small, so that the difference between CK 5 and CK 6 was not apparent (A). In addition, it was difficult to determine whether the central duct epithelial cells were stained in both CK 5 and CK 6 (A). However, in rats, CK 5 was stained in the epithelium and lobules of the duct, and CK 6 was stained only in the ductal epithelial cells (B). In rabbits, the difference between CK 5 and CK 6 was most obvious (C) (A: Mouse, B: Rat, C: Rabbit) (Left: CK 5, Right: CK 6, ×100). |
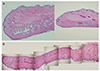 | Figure 5Structures of meibomian glands in different section. In sagittal section (A), although there is an advantage of observing the entire meibomian gland, there is a disadvantage in that it cannot confirm the structure of the gland structure because there is difference in the structure depending on the cutting position. On the other hand, in Transverse section (B), only the portion corresponding to the cutting position can be observed, but the relationship between the central tube and the surrounding lobules could be seen more clearly (A: Sagittal section, B: Transverse section, Hematoxylin and Eosin [H&E] stain, ×40). |
References
1. Jester JV, Rife L, Nii D, et al. In vivo biomicroscopy and photography of meibomian glands in a rabbit model of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 1982; 22:660–667.
2. Baum JL. Biomicroscopic study of the glands of meibomius: by R. Tapie. Ann Ocul 210: 637-648. Surv Ophthalmol. 1979; 23:273–274.
3. Killey FP, Minckler DS, Smith RE, Spencer JA. The effect of long term epinephrine in the rabbit eye. In ARVO Abstracts. Invest Ophthalmol Vis Sci. 1980; 19:253.
4. Luna LG. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed. New York: McGraw-Hill Book Co. Inc.;1968. p. 82.
5. Jester JV, Nicolaides N, Smith RE. Meibomian gland studies: histologic and ultrastructural investigations. Invest Ophthalmol Vis Sci. 1981; 20:537–547.
7. Nicolaides N, Kaitaranta JK, Rawdah TN, et al. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981; 20:522–536.
8. Ehlers N. The precorneal film. Biomicroscopical, histologic, and chemical investigations. Acta Ophthol. 1965; Suppl 81. 1–134.
9. Jester JV, Nicolaides N, Kiss-Palvolgyi I, Smith RE. Meibomian gland dysfunction. II. The Role of Kerarinizarion in a rabbit model of MGD. Invest Ophthalmol Vis Sci. 1989; 30:936–945.
10. Jester JV, Rajagopalan S, Rodrigues M. Meibomian gland changes in the rhino (hrrhhrrh) mouse. Invest Ophthalmol Vis Sci. 1988; 29:1190–1194.
11. Mann SJ. Hair loss and cyst formation in hairless and rhino mutant mice. Anat Rec. 1971; 170:485–499.

12. Davies RE, Austin WA, Logani MK. The rhino mutant mouse as an experimental tool. Trans N Y Acad Sci. 1971; 33:680–693.

13. Ohnishi Y, Kohno T. Polycholorinated biphenyl poisoning in monkey eye. Invest Ophthalmol Vis Sci. 1979; 18:981–984.
14. Gutgesell VJ, Stern GA, Hood CI. Histopathology of meibomian gland dysfunction. Am J Ophthalmol. 1982; 94:383–387.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download