Abstract
Purpose
To evaluate the effect of cauterization on extraocular muscle (EOM) fibrosis in rats, and to develop a novel EOM fibrosis model.
Methods
Twenty-four eyes of 12 Sprague Dawley rats were assigned randomly to two groups. We exposed the superior rectus muscle (SRM) and performed thermal injury 2 mm behind the insertion site of the SRM using a cautery device in the experimental group. The thermal injuries were performed twice for 1 second, for a total of 2 seconds. In the control group, the same procedures except the thermal injury were performed. Two weeks after surgery, all eyes were enucleated and stained with hematoxylin and eosin (H&E) and Masson's trichrome (MT).
References
1. Mora JS, Sprunger DT, Helveston EM, Evan AP. Intraoperative sponge 5-fluorouracil to reduce postoperative scarring in abdominal surgery. J AAPOS. 1997; 1:92–7.
2. Cruz OA. Evaluation of mitomycin to limit postoperative abdominal in strabismus surgery. J Pediatr Ophthalmol Strabismus. 1996; 33:89–92.
3. Minguini N, Monteiro de Carvalho KM, Akaishi PM, De Luca IM. Histologic effect of mitomycin C on strabismus surgery in the rabbit. Invest Ophthalmol Vis Sci. 2000; 41:3399–401.
4. Jung KI, Choi JS, Kim HK, Shin SY. Effects of an abdominal growth factor-beta agent (pirfenidone) on strabismus abdominal in rabbits. Curr Eye Res. 2012; 37:770–6.
5. Ozkan SB, Kir E, Culhaci N, Dayanir V. The effect of Seprafilm on adhesions in strabismus surgery-an experimental study. J AAPOS. 2004; 8:46–9.
6. de Carvalho LE, Alves MR, da Silva MA, Gaal Vadas MF. Experimental strabismus surgery using triamcinolone: outcomes and effects on inflammatory response. Arq Bras Oftalmol. 2007; 70:209–15.
7. Frangouli O, Adams GG. The use of amniotic membrane for the management of fibrosis in complex strabismus surgery. Strabismus. 2013; 21:13–22.

8. Kirsch D, Lowen MS, Fialho Cronemberger MF, Sato EH. Amniotic membrane for reducing the formation of adhesions in strabismus surgery: experimental study in rabbits. J Pediatr Ophthalmol Strabismus. 2014; 51:341–7.

9. Kassem RR, Abdel-Hamid MA, Khodeir MM. Effect of lyophi-lized amniotic membrane on the development of adhesions and abdominal after extraocular muscle surgery in rabbits. Curr Eye Res. 2011; 36:1020–7.
10. Lee MJ, Jin SE, Kim CK, et al. Effect of slow-releasing all-transretinoic acid in bioabsorbable polymer on delayed adjustable strabismus surgery in a rabbit model. Am J Ophthalmol. 2009; 148:566–72.

11. Demirel S, Atilla H, Okcu Heper A, Erkam N. Effects of amniotic membrane on wound healing and adhesions in experimental abdominal surgery. Eur J Ophthalmol. 2009; 19:899–904.
12. Cai EZ, Ang CH, Raju A, et al. Creation of consistent burn wounds: a rat model. Arch Plast Surg. 2014; 41:317–24.

13. Yaacobi Y, Hamed LM, Kaul KS, Fanous MM. Reduction of abdominal adhesions secondary to strabismus surgery in rabbits. Ophthalmic Surg. 1992; 23:123–8.
14. Velnar T, Bailey T, Smrkolj V. The wound healing process: an abdominal of the cellular and molecular mechanisms. J Int Med Res. 2009; 37:1528–42.
15. Vindinský B, Gál P, Toporcer T, et al. Histological study of the first seven days of skin wound healing in rats. Acta Vet Brno. 2006; 75:197–202.
16. Tarran S, Langlois NE, Dziewulski P, Sztynda T. Using the abdominal cell infiltrate to estimate the age of human burn wounds: A review and immunohistochemical study. Med Sci Law. 2006; 46:115–26.
Figure 1.
Demonstration of the operation procedure. (A) Exposure of the upper conjunctiva. (B) Subconjunctival injection of 0.1 mL of normal saline. (C) Limbal peritomy from 10 to 2 o'clock. (D) Isolation of the superior rectus muscle. (E) Thermal injury on superior rectus muscle (black arrow).

Figure 2.
Photomicrographs showing inflammation of the superior rectus muscle two weeks after surgery in the rat model (hematoxylin and eosin staining, ×100). (A) Grade 0. (B) Grade 1. (C) Grade 2. (D) Grade 3.

Figure 3.
Bar graphs showing the degree of inflammation of superior rectus muscle two weeks after surgery. The grade of inflammation was statistically different between control and cauterization group (p = 0.002).
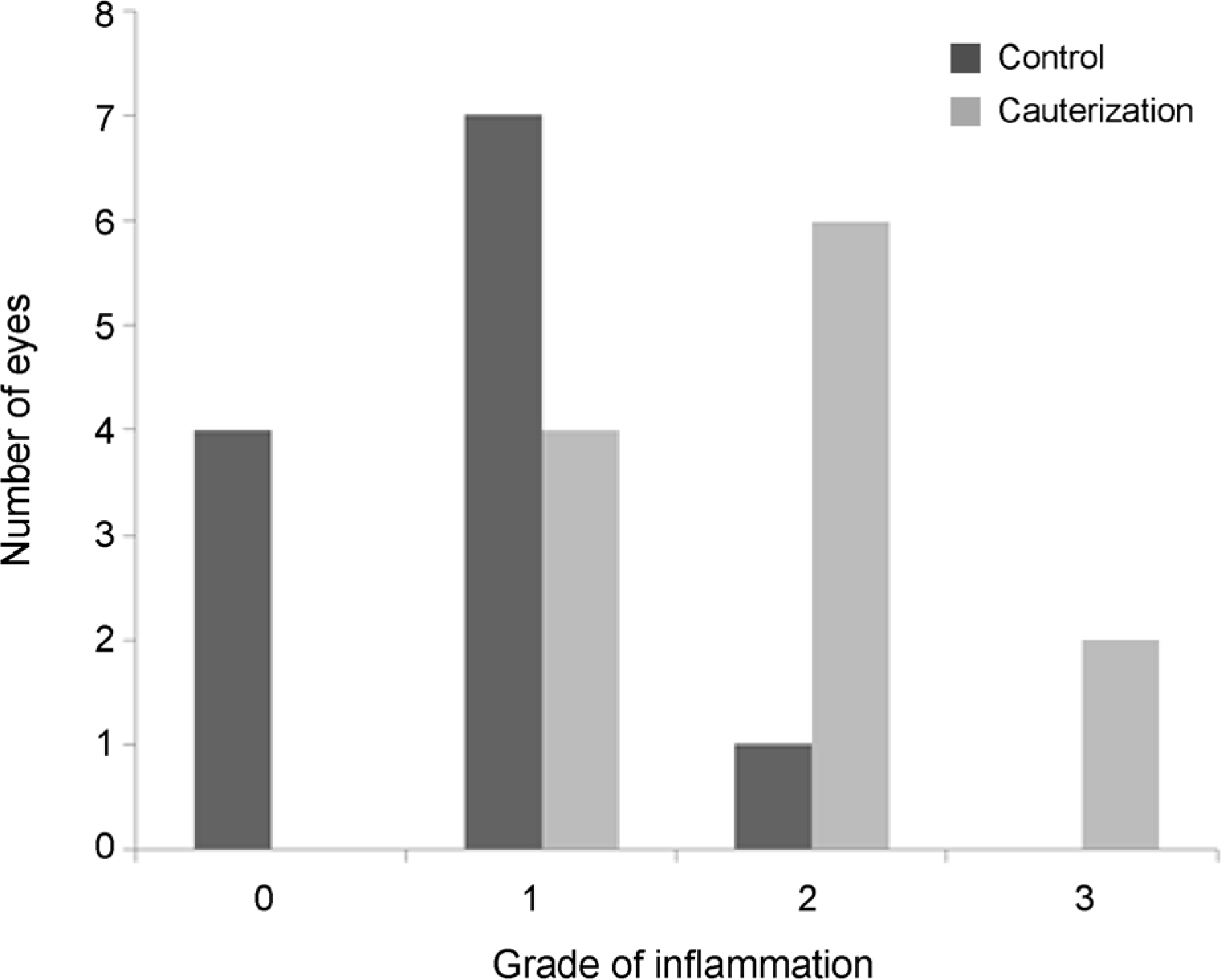
Figure 4.
Photomicrographs showing fibrosis of the superior rectus muscle two weeks after surgery in the rat model (Masson's trichrome staining, ×100). (A) Grade 0. (B) Grade 1. (C) Grade 2. (D) Grade 3. (E) Grade 4.

Figure 5.
Bar graphs showing the degree of fibrosis of superior rectus muscle two weeks after surgery. The grade of fibrosis was statistically different between control and cauterization group (p ≤ 0.001).
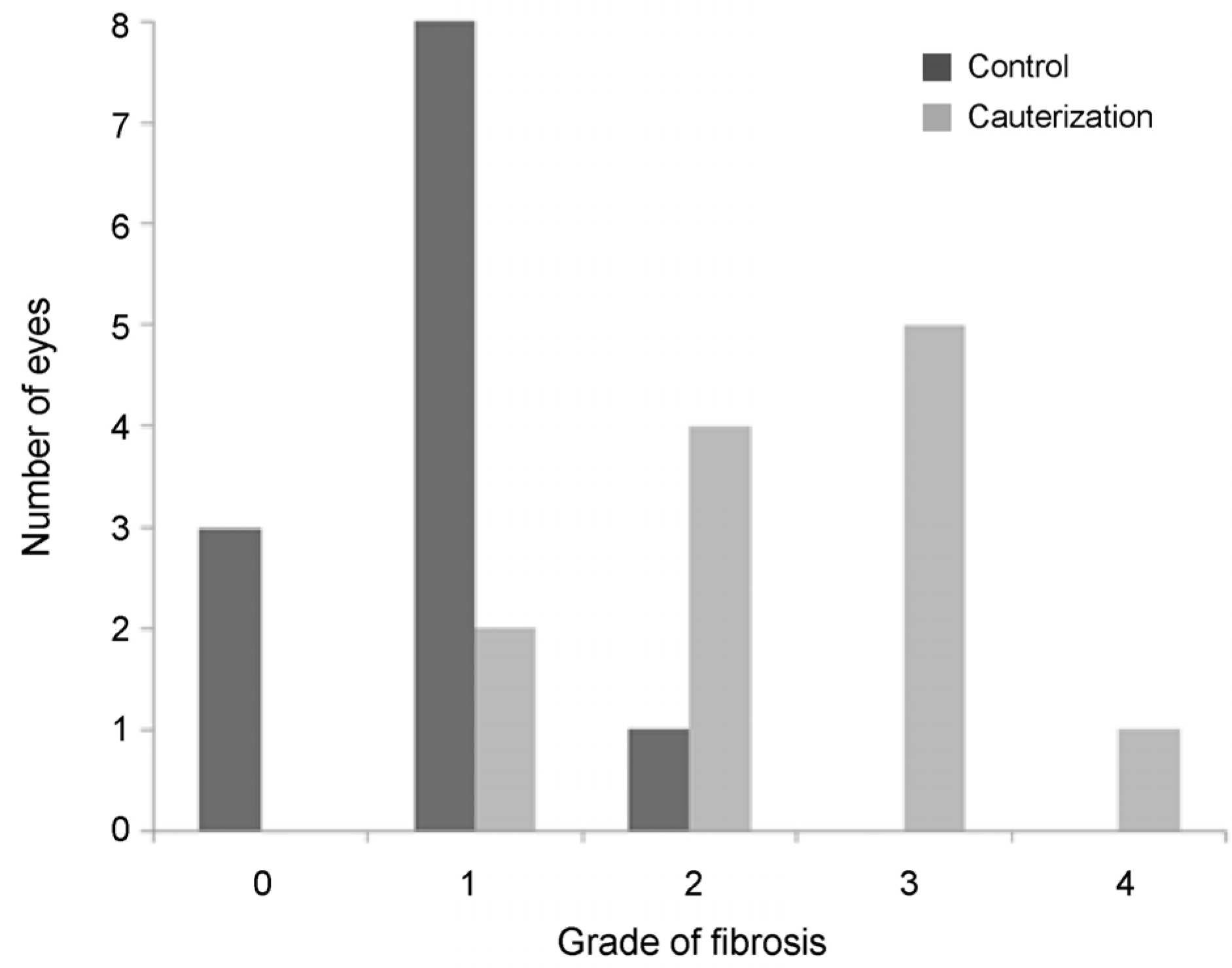
Table 1.
Detailed description of grade of inflammation and fibrosis according to photomicrographic findings




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download