Abstract
Purpose
To compare the allergy prevalence and clinical manifestations of 0.2% brimonidine/0.5% timolol fixed combination (BTFC) and 0.15% brimonidine in Korean patients with glaucoma.
Methods
We retrospectively analyzed the medical records of 196 glaucoma patients treated with BTFC and 234 glaucoma patients treated with 0.15% brimonidine. We compared sex, age, type of glaucoma, treatment period, allergy history, onset time of ocular allergy and clinical characteristics of allergy in the two groups.
Results
Ocular allergy percentages 10.14% in the BTFC group and 22.02% in the 0.15% brimonidine group, and the risk of allergy was approximately 0.4 times lower in patients using BTFC (hazard ratio = 2.5, p = 0.009). The BTFC group developed ocular allergy at a mean of 20.5 months (range: 1.7–51.1 months), and the 0.15% brimonidine group developed ocular allergy at a mean of 7.7 months (range: 0.4–50.8 months). In the BTFC group, 50% of the ocular allergy occurred within 15 months, and within 5 months in the 0.15% brimonidine group. Clinical characteristics of brimonidine allergy involved two types of conjunctival follicles and conjunctival papillae, but there were no significant differences in incidence according to allergy type (p = 0.566).
Go to : 
References
1. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120:701–13. discussion 829–30.
2. Collaborative Normal-Tension Glaucoma Study Group. The abdominalness of intraocular pressure reduction in the treatment of abdominal-tension glaucoma. Am J Ophthalmol. 1998; 126:498–505.
3. Friedman DS, Wilson MR, Liebmann JM, et al. An evidence-based assessment of risk factors for the progression of ocular abdominal and glaucoma. Am J Ophthalmol. 2004; 138(3 Suppl):S19–31.
4. Ahn DH, Lee YG, Hong YJ. Factors affecting compliance with prescribed eyedrops for glaucoma. J Korean Ophthalmol Soc. 1998; 39:2145–51.
5. Yoo SG, Hwang YH. Assessment of glaucoma medication compliance. J Korean Ophthalmol Soc. 2015; 56:365–70.

6. Yeon DY, Yoo C, Park JH, et al. Adherence to preservative-free dorzolamide/timolol fixed combination assessed by counting the unused single-dose units. J Korean Ophthalmol Soc. 2015; 56:906–10.

7. Schuman JS. Clinical experience with brimonidine 0.2% and abdominal 0.5% in glaucoma and ocular hypertension. Surv Ophthalmol. 1996; 41:S27–37.
8. Schuman JS, Horwitz B, Choplin NT, et al. A 1-year study of abdominal twice daily in glaucoma and ocular hypertension. A abdominalled, randomized, multicenter clinical trial. Chronic Brimonidine Study Group. Arch Ophthalmol. 1997; 115:847–52.
9. LeBlanc RP. Twelve-month results of an ongoing randomized trial comparing brimonidine tartrate 0.2% and timolol 0.5% given twice daily in patients with glaucoma or ocular hypertension. Brimonidine Study Group 2. Ophthalmology. 1998; 105:1960–7.
10. Katz LJ. Brimonidine tartrate 0.2% twice daily vs timolol 0.5% twice daily: 1-year results in glaucoma patients. Brimonidine Study Group. Am J Ophthalmol. 1999; 127:20–6.
11. Melanmed S, David R. Ongoing clinical assessment of the safety profile and efficacy of brimonidine compared with timolol: year-three results. Brimonidine Study Group II. Clin Ther. 2000; 22:103–11.
12. Cantor LB. The evolving pharmacotherapeutic profile of abdominal, an alpha 2-adrenergic agonist, after four years of continuous use. Expert Opin Pharmacother. 2000; 1:815–34.
13. Bondeau P, Rousseau JA. Allergic reactions to brimonidine in abdominals treated for glaucoma. Can J Ophthalmol. 2002; 37:21–6.
14. Manni G, Centofanti M, Sacchetti M, et al. Demographic and abdominal factors associated with development of brimonidine tartrate 0.2%-induced ocular allergy. J Glaucoma. 2004; 13:163–7.
15. Stewart WC, Sharpe ED, Harbin TS Jr, et al. Brimonidine 0.2% versus dorzolamide 2% each given three times daily to reduce abdominal pressure. Am J Ophthalmol. 2000; 129:723–7.
16. Katz LJ. Twelve-month evaluation of brimonidine-purite versus brimonidine in patiens with glaucoma or ocular hypertension. J Glaucoma. 2002; 11:119–26.
17. Kim CY, Hong S, Seong GJ. Brimonidine 0.2% versus brimonidine Purite 0.15% in Asian ocular hypertension. J Ocul Pharmacol Ther. 2007; 23:481–6.

18. Goni FJ. 12-week study comparing the fixed combination of abdominal and timolol with concomitant use of the individual abdominal in patients with glaucoma and ocular hypertension. Eur J Ophthalmol. 2005; 15:581–90.
19. Sherwood MB, Craven ER, Chou C, et al. Twice-daily 0.2% brimo-nidine–0.5% timolol fixed-combination therapy vs monotherapy with timolol or brimonidine in patients with glaucoma orocular abdominal: a 12-month randomized trial. Arch Ophthalmol. 2006; 124:1230–8.
20. Motolko MA. Comparison of allergy rates in glaucoma patients abdominal brimonidine 0.2% monotherapy versus fixed-combination brimonidine 0.2%-timolol 0.5% therapy. Curr Med Res Opin. 2008; 24:2663–7.
21. Rosenthal AL, Walters T, Berg E, et al. A comparison of the safety and efficacy of brimonidine 0.2%, BID versus TID, in subjects with elevated intraocular pressure. Invest Ophthalmol Vis Sci. 1996; 37:S1102.
22. Serle JB. A comparison of the safety and efficacy of twice daily abdominal 0.2% versus betaxolol 0.25% in subjects with elevated intraocular pressure. The Brimonidine Study Group III. Surv Ophthalmol. 1996; 41(Suppl 1):S39–47.
23. Derick RJ, Robin AL, Walters TR, et al. Brimonidine tartrate: a one-month dose response study. Ophthalmology. 1997; 104:131–6.
24. Adkins JC, Balfour JA. Brimonidine. A Review of its abdominal properties and clinical potential in the management of open-angle glaucoma and ocular hypertension. Drugs Aging. 1998; 12:225–41.
25. Butler P, Mannschreck M, Lin S, et al. Clinical experience with the long-term use of 1% apraclonidine: incidence of allergic reactions. Arch Ophthalmol. 1995; 113:293–6.
26. Alvarado JA. Reduced ocular allergy with fixed-combination 0.2% brimonidine–0.5% timolol. Arch Ophthalmol. 2007; 125:717. abdominal reply 717–8.

27. Duffin RM, Pettit TH, Straatsma BR. 2.5% vs 10% phenylephrine in maintaining mydriasis during cataract surgery. Arch Ophthalmol. 1983; 101:1903–6.
28. Patil PM, Jacobowitz D. Unequal accumulation of adrenergic drugs by pigmented and nonpigmented iris. Am J Ophthalmol. 1974; 78:470–7.

29. Acheampong AA, Shackleton M, Tang-Liu DD. Comparative abdominal pharmacokinetics of brimonidine after a single dose application to the eyes of albino and pigmented rabbits. Drug Metab Dispos. 1995; 23:708–12.
Go to : 
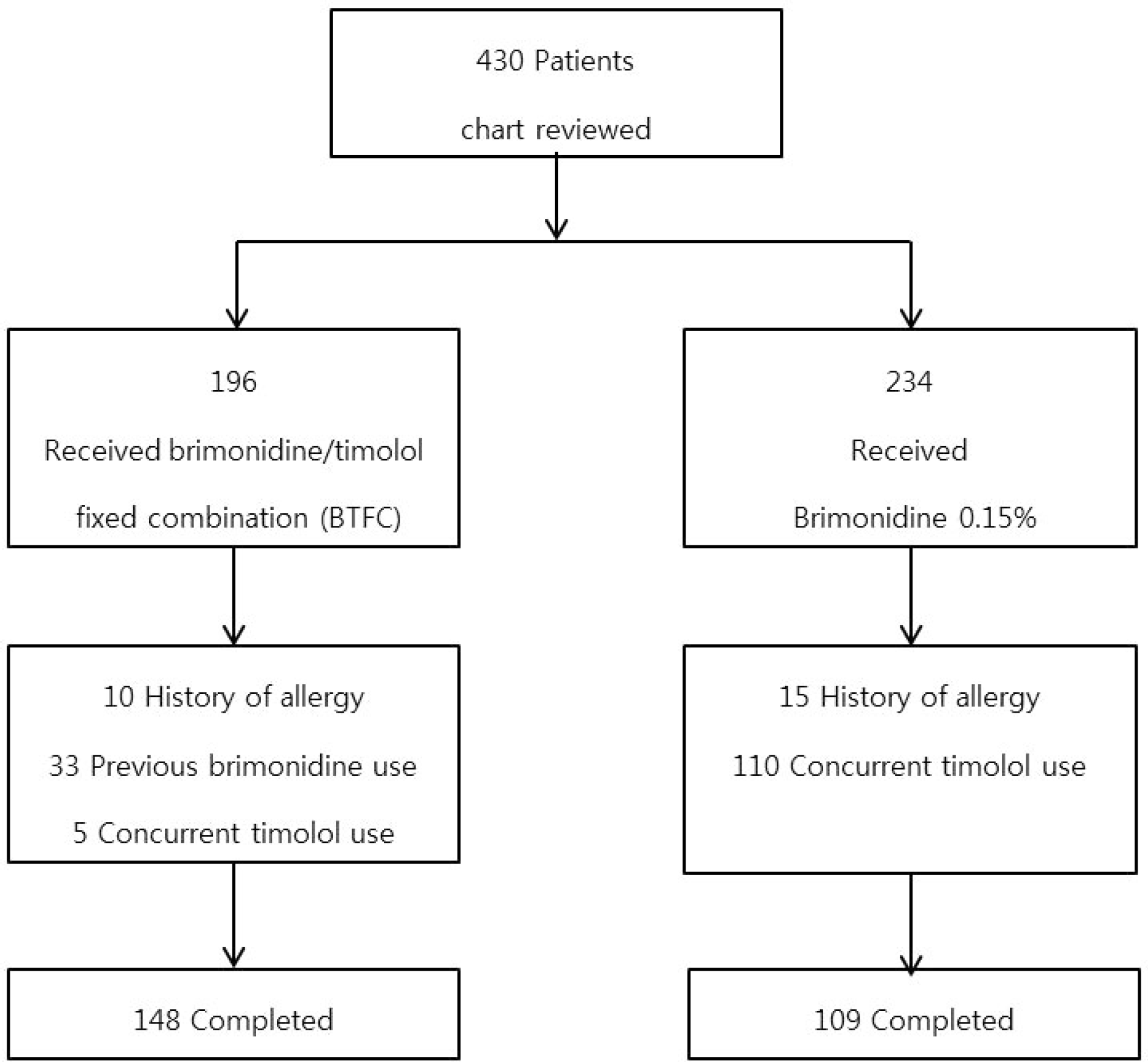 | Figure 1.Summarized flow chart of patients. Among 430 glaucoma patients who treated with brimonidine 0.2%/timolol 0.5% fixed combination (BTFC) or 0.15% brimonidine, a total 257 patients were enrolled after application of exclusion criteria. |
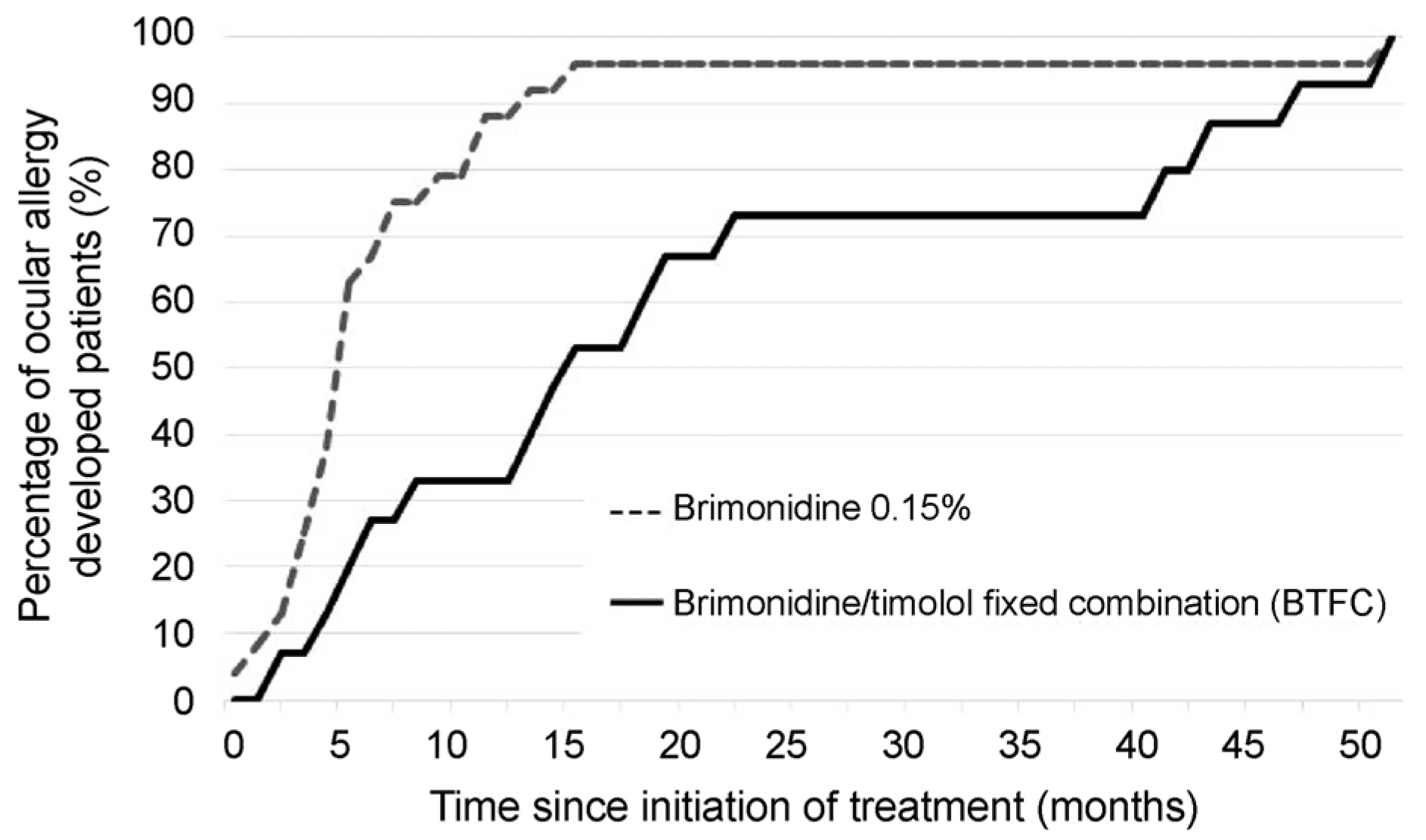 | Figure 2.Percentage of patients who developed an ocular allergy to 0.2% brimonidine/0.5% timolol fixed combination (BTFC) and 0.15% brimonidine. In the BTFC group, 50% of the ocular allergy occurred within 15 months, and within 5 months in the 0.15% brimonidine group. Compared with 0.15% brimonidine group, only 20% of the ocular allergy occurred in the BTFC group within the first 5 months of treatment. |
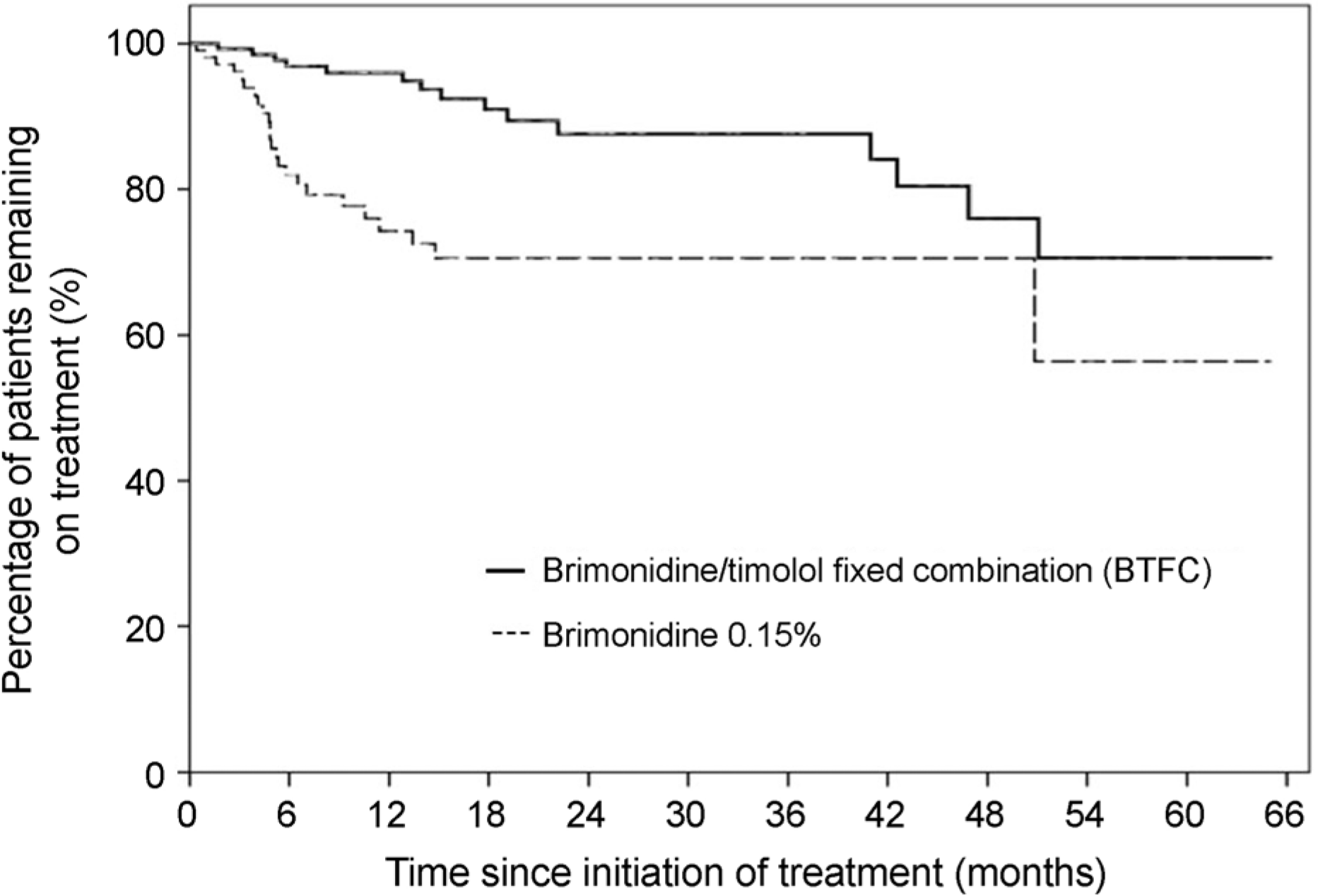 | Figure 3.Kaplan– Meier curve, showing distribution of time to withdrawal due to allergic reaction for 0.2% brimoni-dine/0.5% timolol fixed combination (BTFC) versus 0.15% brimonidine. During the entire course of the observation period, the proportion of patients who discontinued treatment due to ocular allergy was lower in the BTFC group than in the 0.15% brimonidine group. |
 | Figure 4.Slit-lamp photographs of the ocular allergy on left eye in patient who used 0.2% brimonidine/0.5% timolol fixed combination just in her left eye. (A, B) Right eye showed clear cornea and conjunctive. (C, D) Left eye showed significant hyperemia and distinguishing giant follicles on the upper and lower palpebral conjunctivae. |
Table 1.
Baseline characteristics of patients
| Brimonidine/timolol fixed combination (n = 148) | 0.15% brimonidine (n = 109) | p-value | |
|---|---|---|---|
| Mean age (years) | 65.24 ± 14.82 | 65.58 ± 14.34 | 0.853* |
| Sex (male/female) | 72/80 | 52/57 | 0.256† |
| Type of glaucoma (n, %) | |||
| Primary open-angle | 10 (6.76) | 10 (9.17) | |
| Normal tension | 114 (77.03) | 88 (80.73) | 0.246† |
| Primary closed-angle | 7 (4.73) | 6 (5.50) | |
| Others | 17 (11.49) | 5 (4.59) | |
| Follow up time (months) | 20.18 ± 17.13 | 13.70 ± 14.08 | 0.614† |
| Concurrent topical medication (n, %) | |||
| Prostaglandin analogue | 37 (25.00) | 17 (15.60) | 0.088† |
| Carbonic anhydrase inhibitor | 14 (9.50) | 4 (3.67) | 0.086† |
| Pirenoxine | 7 (4.73) | 7 (6.42) | 0.587† |
| Artificial tear | 59 (39.86) | 34 (3.19) | 0.189† |
| Cyclosporine | 4 (2.70) | 4 (3.67) | 0.726† |
| Acyclovir ointment | – | 2 (1.83) | 0.179† |
| Concurrent ocular surface disease (n, %) | |||
| Dry eye syndrome | 60 (40.54) | 37 (33.94) | 0.300† |
| Herpes keratitis | – | 2 (1.83) | 0.179† |
Table 2.
Onset of allergy and rate of ocular allergic reaction and systemic adverse event
| Brimonidine/timolol fixed combination (n = 148) | 0.15% brimonidine (n = 109) | p-value* | HR | 95% CI | |
|---|---|---|---|---|---|
| Onset of allergy (months) | 20.47 ± 16.71 (1.73–51.07) | 7.66 ± 9.91 (0.40–50.83) | |||
| Ocular allergy (n, %) | 15 (10.14) | 24 (22.02) | 0.009 | 2.50 | 1.24–5.04 |
| Blepharoconjunctivitis | 2 | 4 | |||
| Conjunctival follicles | 9 | 15 | 0.566 | ||
| Conjunctival papillae | 6 | 9 | |||
| Systemic adverse event (n) | 1 | 5 | 0.040 | 7.07 | 0.81–61.38 |
Table 3.
Change of glaucoma medication classes after onset of ocular allergic reaction




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download