Abstract
Purpose
To evaluate the clinical differences between patients with central serous chorioretinopathy (CSC) developed after steroid use and CSC patients without a history of steroid use for short-term periods.
Methods
We retrospectively analyzed the medical records of 47 patients (55 eyes) diagnosed with CSC from January 2011 to August 2017 by categorizing Group 1 (32 patients, 36 eyes) without a history of steroid use and Group 2 (15 patients, 19 eyes) with a history of steroid use within 6 months. We evaluated the differences in best-corrected visual acuity (BCVA), subretinal fluid (SRF) height, subfoveal choroidal thickness (SFCT), and Haller's layer thickness in the two groups. We also analyzed the changes in the BCVA, SRF height, SFCT, and Haller's layer thickness in each group for 1 month and compared them depending on the treatment.
Results
There were no significant differences between the two groups with regard to age, sex, BCVA, bilaterality, number of leakage points, and Haller's layer thickness. Group 2 showed significantly increased SRF height and SFCT than Group 1 (p = 0.002, p = 0.005, respectively). In Group 1, the level of SRF and SFCT were significantly more decreased after 1 month (p = 0.001, 0.015, respectively) in patients with treatment than in those without treatment. In Group 2, the height of the SRF and SFCT were significantly more decreased after 1 month (p = 0.005, 0.002, respectively) in untreated patients compared to treated patients.
Go to : 
REFERENCES
1). Spitznas M. Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol. 1986; 224:321–4.

2). Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967; 63(Suppl):1–139.
3). Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002; 47:431–48.

4). Karadimas P, Bouzas EA. Glucocorticoid use represents a risk factor for central serous chorioretinopathy: a prospective, case-control study. Graefes Arch Clin Exp Ophthalmol. 2004; 242:800–2.

5). Miki A, Ikuno Y, Jo Y, Nishida K. Comparison of enhanced depth imaging and high-penetration optical coherence tomography for imaging deep optic nerve head and parapapillary structures. Clin Ophthalmol. 2013; 7:1995–2001.

6). Park HY, Shin HY, Park CK. Imaging the posterior segment of the eye using swept-source optical coherence tomography in myopic glaucoma eyes: comparison with enhanced-depth imaging. Am J Ophthalmol. 2014; 157:550–7.

7). Chung YR, Kim JW, Choi SY, et al. Subfoveal choroidal thickness and vascular diameter in active and resolved cental serous chorioretinopathy. Retina. 2018:38:102–7.
8). Lee WJ, Lee JW, Park SH, Lee BR. En face choroidal vascular feature imaging in acute and chronic central serous chorioretinopathy using swept source optical coherence tomography. Br J Ophthalmol. 2017; 101:580–6.

9). Bansal P, Agarwal A, Gupta V, et al. Sprectral domain optical coherence tomography changes following intravitreal dexamethasone implant, Ozurdex(R) in patients with uveitic cystoid macular edema. Indian J Ophthalmol. 2015; 63:416–22.
10). Staurenghi G, Sadda S, Chakravarthy U, et al. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the INOCT consensus. Ophthalmology. 2014; 121:1572–8.
11). Branchini LA, Adhi M, Regatieri CV, et al. Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology. 2013; 120:1901–8.

12). Gelber GS, Schatz H. Loss of vision due to central serous chorioretinopathy following psychological stress. Am J Psychiatry. 1987; 144:46–50.
13). Quillen DA, Gass DM, Brod RD, et al. Central serous chorioretinopathy in women. Ophthalmology. 1996; 103:72–9.

14). Loo JL, Lee SY, Ang CL. Can long-term corticosteroids lead to blindness? A case series of central serous chorioretinopathy induced by corticosteroids. Ann Acad Med Singapore. 2006; 35:496–9.
15). Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146:496–500.

16). Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009; 147:811–5.

17). Jain IS, Singh K. Maculopathy a corticosteroid side-effect. J All India Ophthalmol Soc. 1966; 14:250–2.
18). Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010; 117:1792–9.

19). Esmaeelpour M, Ansari-Shahrezaei S, Glittenberg C, et al. Choroid, Haller's, and Sattler's layer thickness in intermediate age-related macular degeneration with and without fellow neovascular eyes. Invest Ophthalmol Vis Sci. 2014; 55:5074–80.

20). Spaide RF, Campeas L, Haas A, et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology. 1996; 103:2070–9. discussion 2079-80.

21). Ficker L, Vafidis G, While A, Leaver P. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol. 1988; 72:829–34.

22). Watzke RC, Burton TC, Woolson RF. Direct and indirect laser photocoagulation of central serous choroidopathy. Am J Ophthalmol. 1979; 88:914–8.

23). Artunay O, Yuzbasioglu E, Rasier R, et al. Intravitreal bevacizumab in treatment of idiopathic persistent central serous chorioretinopathy: a prospective, controlled clinical study. Curr Eye Res. 2010; 35:91–8.

24). Seong HK, Bae JH, Kim ES, et al. Intravitreal bevacizumab to treat acute central serous chorioretinopathy: short-term effect. Ophthalmologica. 2009; 223:343–7.

25). Benson SE, Schlottmann PG, Bunce C, et al. Optical coherence tomography analysis of the macular after scleral buckle surgery for retinal detachment. Ophthalmology. 2007; 114:108–12.
26). Sohn EH, Khanna A, Tucker BA, et al. Structural and biochemical analyses of choroidal thickness in human donor eyes. Invest Ophthalmol Vis Sci. 2014; 55:1352–60.

27). Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography ofthe choroid in central serous chorioretinopathy. Retina. 2009; 29:1469–73.
28). Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol. 2013; 58:387–429.

29). Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999; 128:63–8.

Go to : 
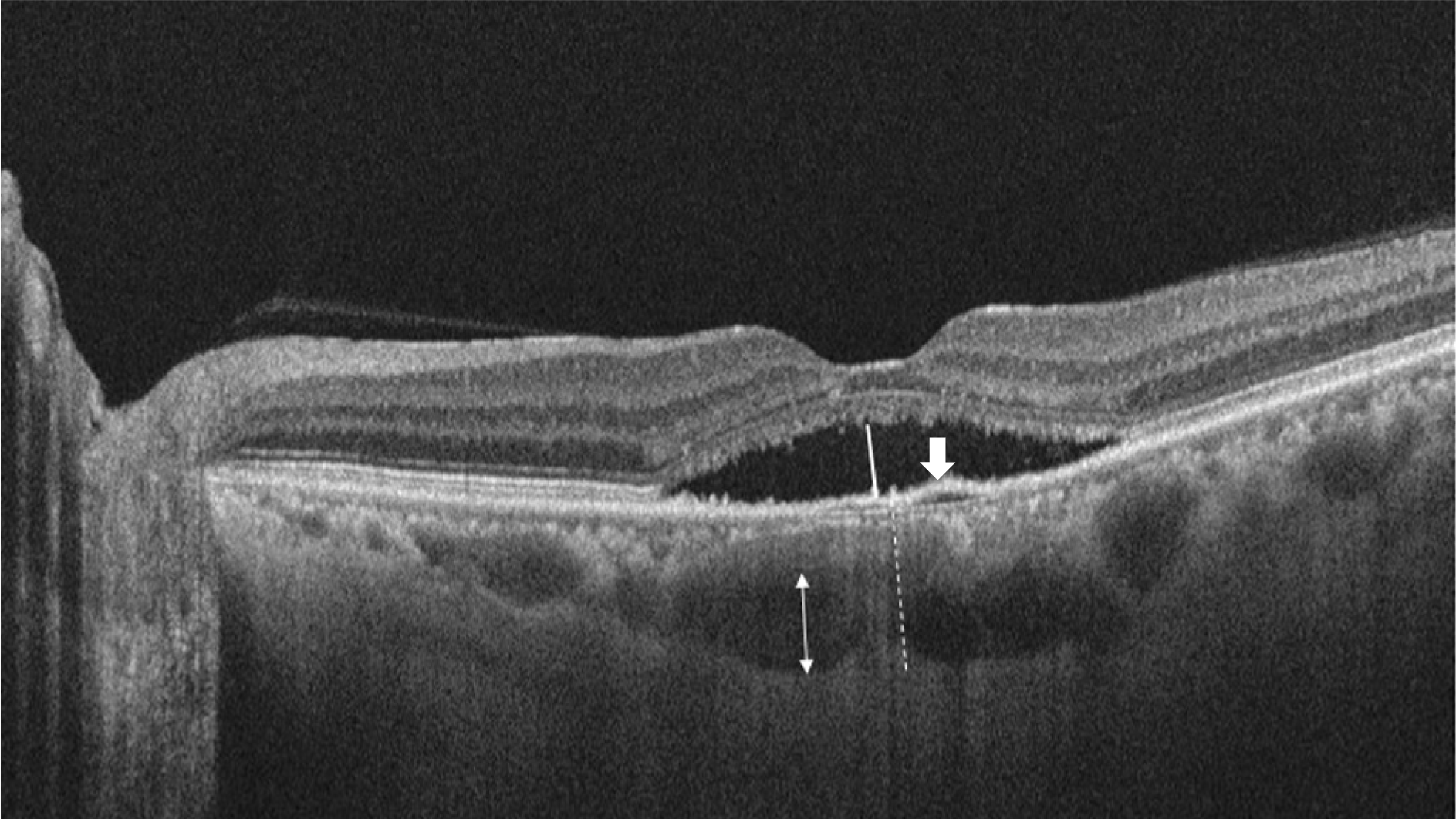 | Figure 1.Optical coherence tomography scan image of a patient. Height of serous retinal detachment was manually measured using calipers on the software as shown by the white line. The dotted line shows subfoveal choroid thickness that represent from outer border of retinal pigment epithelium to inner border of sclera. The white arrow denotes pigment epithelial detachment. The thickness of Haller's layer was measured from the inner border of the choroid-sclera junction to the innermost point of the selected large choroidal vessel at the subfoveal location (white double-headed arrow). |
Table 1.
Clinical demographics in each group
| Group 1 (n = 36) | Group 2 (n = 19) | p-value | |
|---|---|---|---|
| Age (years) | 55.12 ± 9.00 | 51.87 ± 11.44 | 0.368* |
| Sex (male:female) | 26:6 | 13:2 | 0.341† |
| Bilaterality | 4 | 4 | 0.523† |
| SRF height (μm) | 122.39 ± 172.42 | 225.69 ± 123.58 | 0.002* |
| Choroidal thickness (μm) | 379.64 ± 103.28 | 477.05 ± 136.08 | 0.005* |
| Haller's layer thickness (μm) | 215.56 ± 56.07 | 230.44 ± 112.18 | 0.453* |
| Leaking point | 1.28 ± 0.62 | 1.63 ± 1.01 | 0.080* |
| Relapse | 6 | 5 | 0.375‡ |
| Chronicization | 7 | 2 | 0.473‡ |
Table 2.
Clinical features of steroid induced central serous chorioretinopathy (CSC)
Table 3.
Correlation between subfoveal choroidal thickness and subretinal detachment height in two groups
| Choroidal thickness (μm) | SRF height (μm) | p-value* | Correlation coefficient | |
|---|---|---|---|---|
| Group 1 | 379.64 ± 103.28 | 122.39 ± 172.42 | 0.972 | −0.006 |
| Group 2 | 477.05 ± 136.08 | 225.69 ± 123.58 | 0.819 | −0.056 |
Table 4.
Comparison of visual acuity at baseline and 1 month later in two groups
|
Group 1 (n = 36) |
Group 2 (n = 19) |
p-value* | |||
|---|---|---|---|---|---|
| BCVA (logMAR) | Improved BCVA | BCVA (logMAR) | Improved BCVA | ||
| Baseline BCVA | 0.24 ± 0.25 | 0.19 ± 0.24 | 0.482 | ||
| BCVA after 1 month | 0.26 ± 0.25 | 0.265† | 0.14 ± 0.13 | 0.242† | 0.130 |
Table 5.
Comparison of visual acuity, subfoveal choroidal thickness, Haller's layer thickness, subretinal detachment height at baseline and 1 month after in non steroidal central serous chorioretinopathy (CSC) group
| Treated (n = 24) | Observation (n = 12) | p-value | ||
|---|---|---|---|---|
| BCVA | Initial | 0.27 ± 0.24 | 0.19 ± 0.28 | 0.255† |
| 1 month later | 0.25 ± 0.22 | 0.27 ± 0.32 | 0.705† | |
| p-value | 0.428* | 0.102* | ||
| Choroidal thickness (μm) | Initial | 388.08 ± 105.26 | 362.75 ± 101.53 | 0.902† |
| 1 month later | 339.67 ± 91.69 | 338.25 ± 101.27 | 0.987† | |
| p-value | 0.001* | 0.557* | ||
| Haller's layer thickness (μm) | Initial | 205.86 ± 54.28 | 233.33 ± 57.20 | 0.159† |
| 1 month later | 181.56 ± 61.73 | 227.61 ± 65.60 | 0.125† | |
| p-value | 0.651* | 0.219* | ||
| SRF height (μm) | Initial | 131.25 ± 185.71 | 77.25 ± 107.08 | 0.121† |
| 1 month later | 47.75 ± 50.34 | 44.92 ± 57.96 | 0.537† | |
| p-value | 0.015* | 0.102* |
Table 6.
Comparison of treatment modality in two groups
| Group 1 (n = 36) | Group 2 (n = 19) | p-value* | |
|---|---|---|---|
| Photocoagulation | 9 | 1 | 0.011 |
| IVB | 5 | 2 | 0.602 |
| PDT | 3 | 0 | 0.545 |
| Combination | 7 | 4 | 0.636 |
| IVB/PDT | 1 | 4 | |
| Photocoagulation/PDT | 2 | 0 | |
| Photocoagulation/IVB | 3 | 0 | |
| Photocoagulation/PDT/IVB | 1 | 0 | |
| Observation | 12 | 12 | 0.049† |
| p-value | 0.087† | 1.0‡ | 0.011‡ |
Table 7.
Comparison of visual acuity, subfoveal choroidal thickness, Haller's layer thickness, subretinal detachment height at baseline and 1 month after in steroid induced central serous chorioretinopathy (CSC) group
| Treated (n = 7) | Observation (n = 12) | p-value | ||
|---|---|---|---|---|
| BCVA | Initial | 0.34 ± 0.32 | 0.11 ± 0.12 | 0.055† |
| 1 month later | 0.14 ± 0.11 | 0.13 ± 0.14 | 0.789† | |
| p-value | 0.250* | 0.437* | ||
| Choroidal thickness (μm) | Initial | 393.29 ± 101.36 | 525.92 ± 132.77 | 0.018† |
| 1 month later | 352.14 ± 128.82 | 426.75 ± 144.46 | 0.261† | |
| p-value | 0.203* | 0.005* | ||
| Haller's layer thickness (μm) | Initial | 197.50 ± 57.85 | 246.92 ±130.53 | 0.437† |
| 1 month later | 172.47 ± 64.48 | 231.27 ± 53.15 | 0.251† | |
| p-value | 0.451* | 0.519* | ||
| SRF height (μm) | Initial | 191.71 ± 143.87 | 172.33 ± 125.03 | 0.853† |
| 1 month later | 127.14 ± 125.46 | 42.25 ± 77.82 | 0.094† | |
| p-value | 0.375* | 0.002* |
Table 8.
Comparison of baseline demographics following steroid administered in steroid induced central serous chorioretinopathy
|
Steroid-induced CSC |
p-value | ||
|---|---|---|---|
| Oral intake (n = 15) | Injection (n = 4) | ||
| Age (years) | 52.64 ± 12.27 | 49.75 ± 10.05 | 0.551* |
| Sex (male:female) | 11:1 | 3:1 | 0.450† |
| Baseline BCVA (logMAR) | 0.21 ± 0.25 | 0.16 ± 0.15 | 0.250* |
| 1 month later BCVA (logMAR) | 0.16 ± 0.15 | 0.15 ± 0.17 | 0.515* |
| SRF height (μm) | 264.89 ± 124.00 | 137.50 ± 71.18 | 0.099‡ |
| Bilaterality | 5 | 0 | 0.181† |
| Leaking point | 1.62 ± 1.03 | 1.50 ± 1.00 | 0.738† |
| Choroidal thickness (μm) | 465.38 ± 123.79 | 542.50 ± 172.99 | 0.740‡ |
| Haller's layer thickness (μm) | 245.93 ± 105.67 | 181.00 ± 123.03 | 0.203‡ |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download