Abstract
Purpose
To evaluate microaneurysm (MA) turnover and changes in central retinal thickness after intravitreal dexamethasone implantation or intravitreal bevacizumab injection to treat diabetic macular edema.
Methods
Sixty eyes with diabetic macular edema were evaluated. In all, 30 eyes received intravitreal dexamethasone implants (group A) and 30 received bevacizumab injections (group B). All patients were followed-up at 3 and 6 months. MA formation, disappearance, and turnover (MA formation rate minus disappearance rate) were evaluated. When the disappearance rate was greater than the formation rate (so the turnover was 30), the microaneurysms were considered to have resolved. Central retinal thickness (CRT) was measured using optical coherence tomography at all visits.
Results
In group A, MA turnover was 86.6% at 3 months and 53.3% at 6 months, and thus decreased slightly over time, but was not eliminated. In group B, MA turnover was 56.6% at 3 months and 13.3% at 6 months; the between-group difference was statistically significant (p = 0.014). CRT decreased in both groups, but significantly less so in group B 3 months after injection. However, no significant between-group difference was apparent 6 months after injection.
Conclusions
There were no significant between-group differences in either CRT or MA turnover 3 months after injection. However, at 6 months, dexamethasone implantation showed slightly better results than intravitreal bevacizumab injection. However, further research on long-term MA turnover is required.
Go to : 
REFERENCES
1). Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998; 21(Suppl 3):C11–4.

2). Kim HK, Oh TS, Lee SM, Lee JB. The initial fundus examinationand severity of diabetic retinopathy at a primary eye clinic. J Korean Ophthalmol Soc. 2005; 46:982–8.
3). Arevalo JF, Garcia-Amaris RA. Intravitreal bevacizumab for diabetic retinopathy. Curr Diabetes Rev. 2009; 5:39–46.

4). Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011; 52:80–6.

5). Han HC, Bang JW, Yum JH, et al. A case of acute endophthalmitis following a dexamethasone intravitreal implant. J Korean Ophthalmol Soc. 2013; 54:1939–44.

6). Kuppermann BD, Blumenkranz MS, Haller JA, et al. Randomized controlled study of an intravitreous dexamethasone drug deliverysystem in patients with persistent macular edema. Arch Ophthalmol. 2007; 125:309–17.
7). Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–46.e3.

8). Haritoglou C, Kook D, Neubauer A, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006; 26:999–1005.

9). Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007; 114:743–50.
10). Nunes S. Ribeiro L, Lobo C, Cunha-Vaz J. Three different phenotypes of mild nonproliferative diabetic retinopathy with different risks for development of clinically significant macular edema. Invest Ophthalmol Vis Sci. 2013; 54:4595–604.
11). Cunha-Vaz J. Characterization and relevanceof different diabetic retinopathy phenotypes. Dev Ophthalmol. 2007; 39:13–30.
12). Nunes S, Pires I, Rosa A, et al. Microaneurysm turnover is a biomarker for diabetic retinopathy progression to clinically significant macular edema: findings for type 2 diabetics with nonproliferative retinopathy. Ophthalmologica. 2009; 223:292–7.

13). Kohner EM, Stratton IM, Aldington SJ, et al. Microaneurysms in the development of diabetic retinopathy (UKPDS 42). UK Prospective Diabetes Study Group. Diabetologia. 1999; 42:1107–12.
14). Klein R, Meuer SM, Moss SE, Klein BE. The relationship of retinal microaneurysm counts to the 4-year progression of diabetic retinopathy. Arch Ophthalmol. 1989; 107:1780–5.

15). Haritoglou C, Kernt M, Neubauer A, et al. Microaneurysm formation rate as a predictive marker for progression to clinically significant macular edema in nonproliferative diabetic retinopathy. Retina. 2014; 34:157–64.

16). Choi CW, Lee SJ, Kang HR, Yang YS. The change of microaneurysm in diabetic retinopathy patients who undergo intravitreal avastin (bevacizumab) injection. J Korean Ophthalmol Soc. 2014; 55:1481–6.

17). Leicht SF, Kernt M, Neubauer A, et al. Microaneurysm turnover in diabetic retinopathy assessed by automated RetmarkerDR image analysis–potential role as biomarker of response to ranibizumab treatment. Ophthalmologica. 2014; 231:198–203.
18). Stitt AW, Gardiner TA, Archer DB. Histological and ultrastructural investigation of retinal microaneurysm development indiabetic patients. Br J Ophthalmol. 1995; 79:362–7.
19). Ribeiro ML, Nunes SG, Cunha-Vaz JG. Microaneurysm turnoverat the macula predicts risk of development of clinically significant macular edema in persons with mild nonproliferative diabetic retinopathy. Diabetes Care. 2013; 36:1254–9.
20). Sohn HJ, Han DH, Kim IT, et al. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011; 152:686–94.

21). Pardo-Lopez D, Francés-Munoz E, Gallego-Pinazo R, Diaz-Llopis M. Anterior chamber migration of dexametasone intravitreal implant (Ozurdex(R)). Graefes Arch Clin Exp Ophthalmol. 2012; 250:1703–4.
22). Aroney C, Fraser-Bell S, Lamoureux EL, et al. Invest. Ophthalmol Vis Sci. 2016; 57:5541–6.
Go to : 
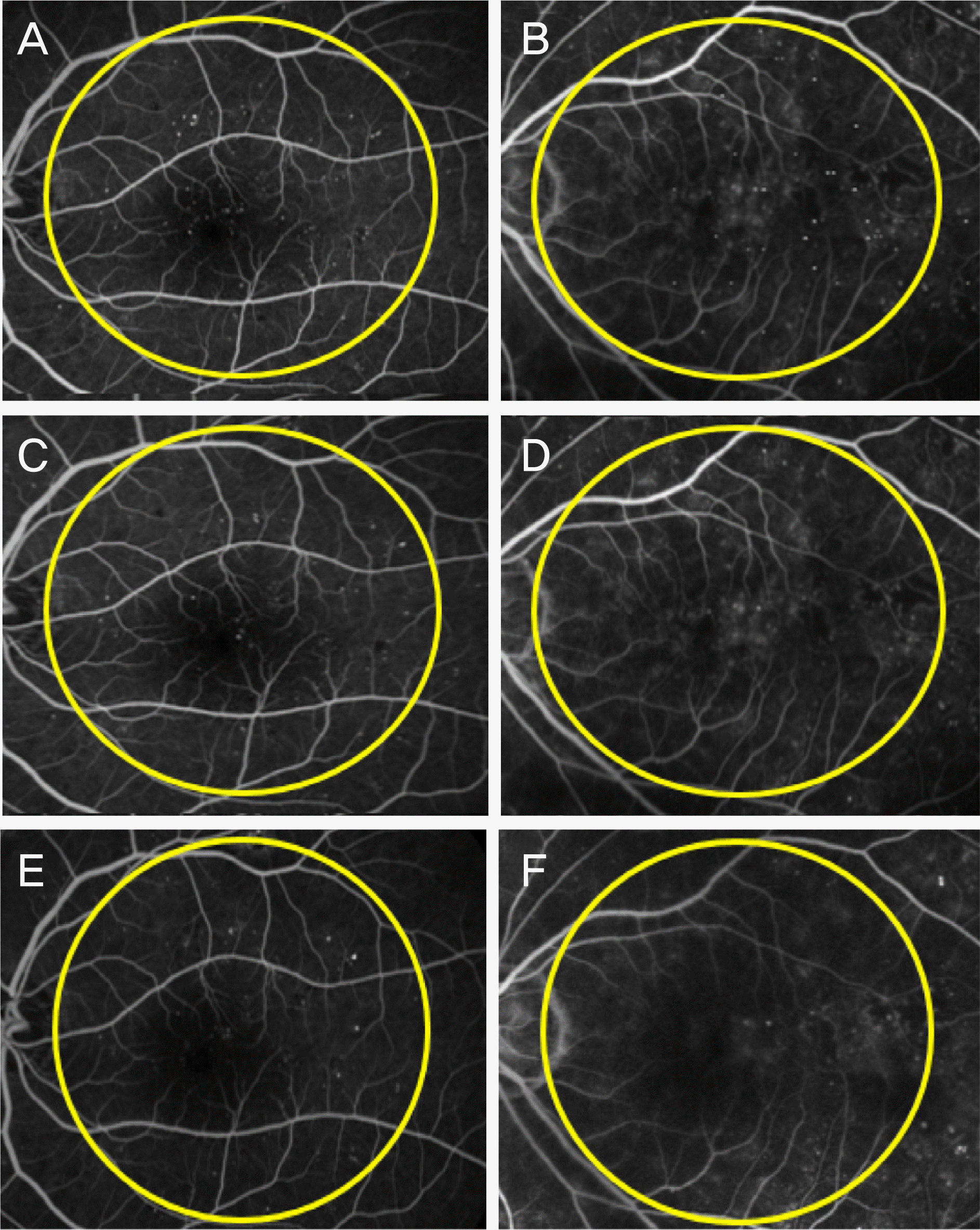 | Figure 1.The Microaneurysm of pre-injectional state (A, B) and at 3 and 6 months post-injectional state (C, D and E, F). The yellow circle is in radius of optic disc and macula (A, C, E = dexamethasone implant) (B, D, F = bevacizumab injection). |
Table 1.
Patients demographics
| Dexamethasone implant | Bevacizumab | p-value | |
|---|---|---|---|
| Study population (n) | 30 | 30 | |
| Male:Female (n) | 18:12 | 16:14 | 0.591 |
| Mean age (years) | 68.80 ±7.18 | 62.30 ±7.92 | 0.161* |
| Mean baseline BCVA (logMAR) | 0.46 ±0.53 | 0.37 ±0.28 | 0.579* |
| Mean baseline CMT (pm) | 403.85 ± 114.61 | 459.08 ± 104.90 | 0.243* |
| Initial number of MAs | 33.42 ±3.92 | 32.75 ±2.81 | 0.131* |
Table 2.
Best corrected visual acuity
| Baseline | 3 months | 6 months | |
|---|---|---|---|
| Dexamethasone implant | 0.46 ±0.53 | 0.35 ±0.39 | 0.23 ± 0.40 |
| Bevacizumab | 0.27 ±0.28 | 0.33 ±0.26 | 0.19 ±0.15 |
| p-value | 0.547 | 0.186 | 0.419 |
Table 3.
Central macular thickness (μm)
| Baseline | 3 months | 6 months | |
|---|---|---|---|
| Dexamethasone implant | 403.85 ± 114.61 | 264.25 ± 29.22 | 306.10 ± 32.27 |
| Bevacizumab | 459.08 ± 104.90 | 324.15 ± 64.85 | 334.56 ± 92.79 |
| p-value | 0.054 | 0.048* | 0.122 |
Table 4.
Number of microaneurysms
| Baseline | 3 months | 6 months | |
|---|---|---|---|
| Dexamethasone implant | 33.42 ± 3.92 | 16.23 ± 4.12 | 18.52 ± 2.94 |
| Bevacizumab | 32.75 ± 2.81 | 21.25 ± 5.22 | 25.25 ± 3.02 |
| p-value | 0.169 | 0.048* | 0.032* |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download