Abstract
Purpose
To evaluate long-term treatment outcomes of intravitreal aflibercept monotherapy for polypoidal choroidal vasculopathy (PCV).
Methods
A retrospective review of medical records was performed with 46 patients who were diagnosed with PCV and treated with aflibercept monotherapy for 24 months. Best-corrected visual acuity (BCVA) values measured at diagnosis, 3 months, 12 months, and 24 months were compared. Baseline morphological factors associated with the 24 month BCVA were additionally investigated.
Results
The mean age of the patients was 65.8 ± 7.9 years. The patients were treated with a mean of 7.0 ± 2.3 aflibercept injections. The mean logarithm of the minimal angle of resolution (logMAR) BCVA at diagnosis, 3 months, 12 months, and 24 months was 0.56 ± 0.40, 0.36 ± 0.36, 0.45 ± 0.42, and 0.52 ± 0.47, respectively. When compared with baseline values, the BCVA was significantly improved at 3 months (p < 0.001) and 12 months (p = 0.022). However, the value at 24 months was not significantly different (p = 1.000). The BCVA was improved or maintained in 35 eyes (76.1%). Extrafoveal polypoidal lesions were associated with a better 24 month visual outcome than subfoveal/juxtafoveal lesions.
Go to : 
REFERENCES
1). Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008; 115:116–26.
2). Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

3). Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–44.

4). Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multi-center cohort study (SEVEN-UP). Ophthalmology. 2013; 120:2292–9.
5). Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–48.

6). Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 2013; 97:1032–5.

7). Kim JH, Cho NC, Kim WJ. Intravitreal aflibercept for neovascular age-related macular degeneration resistant to bevacizumab and ranibizumab. J Korean Ophthalmol Soc. 2015; 56:1359–64.

8). Cho HJ, Kim KM, Kim HS, et al. Intravitreal aflibercept and ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2016; 165:1–6.

9). Park KH, Song SJ, Lee WK, et al. The results of nation-wide registry of age-related macular degeneration in Korea. J Korean Ophthalmol Soc. 2010; 51:516–23.
10). Lee D, Jeong S, Moon J, et al. Analysis of efficacy of intravitreal aflibercept according to subfoveal choroidal thickness in polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2016; 57:1577–85.

11). Yang H, Jeon HM, Kim SW, et al. Short-term efficacy of intravitreal aflibercept for polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2015; 56:1728–35.

12). Kim IG, Kim YI, Kim JS, et al. Comparison of choroidal thickness change between ranibizumab and aflibercept in age-related macular degeneration: six month results. J Korean Ophthalmol Soc. 2017; 58:296–304.

13). Kawamura A, Yuzawa M, Mori R, et al. Indocyanine green angiographic and optical coherence tomographic findings support classification of polypoidal choroidal vasculopathy into two types. Acta Ophthalmol. 2013; 91:e474–81.

14). Yuzawa M, Mori R, Kawamura A. The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol. 2005; 89:602–7.

15). Coscas G, Yamashiro K, Coscas F, et al. Comparison of exudative age-related macular degeneration subtypes in Japanese and French patients: multi-center diagnosis with multimodal imaging. Am J Ophthalmol. 2014; 158:309–18.e2.

16). Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011; 118:840–5.

17). Kim JH, Chang YS, Lee TG, Kim CG. Choroidal vascular hyperpermeability and punctate hyperfluorescent spot in choroidal neovascularization. Invest Ophthalmol Vis Sci. 2015; 56:1909–15.

18). Ma L, Li Z, Liu K, et al. Association of genetic variants with polypoidal choroidal vasculopathy: a systematic review and updated meta-analysis. Ophthalmology. 2015; 122:1854–65.
19). Oishi A, Kojima H, Mandai M, et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy: 12-month LAPTOP study results. Am J Ophthalmol. 2013; 156:644–51.

20). Lee MH, An JH, Lee JE, Oum BS. Short-term efficacy of intravitreal bavacizumab for polypoidal choroidal vasculopathy. J Korean Ophthalmol Soc. 2009; 50:51–60.

21). Koh AH; Expert PCV Panel, Chen LJ, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina. 2013; 33:686–716.
22). Kim JH, Lee TG, Chang YS, et al. Short-term choroidal thickness changes in patients treated with either ranibizumab or aflibercept: a comparative study. Br J Ophthalmol. 2016; 100:1634–9.

23). Maruyama-Inoue M, Sato S, Yamane S, Kadonosono K. Intravitreal injection of aflibercept in patients with polypoidal choroidal vasculopathy: a 3-year follow-up. Retina. 2017; Aug. 14. DOI: 10.1097/IAE.0000000000001818. [Epub ahead of print].
24). Morimoto M, Matsumoto H, Mimura K, Akiyama H. Two-year results of a treat-and-extend regimen with aflibercept for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2017; 255:1891–7.

25). Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–83.

26). Freund KB, Korobelnik JF, Devenyi R, et al. Treat-and-extend regimens with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina. 2015; 35:1489–506.
27). Hikichi T, Kitamei H, Shioya S. Prognostic factors of 2-year outcomes of ranibizumab therapy for polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015; 99:817–22.

28). Chang YS, Kim JH, Kim JW, et al. Polypoidal choroidal vasculopathy in patients aged less than 50 years: characteristics and 6-month treatment outcome. Graefes Arch Clin Exp Ophthalmol. 2016; 254:1083–9.
29). Kang HM, Koh HJ. Long-term visual outcome and prognostic factors after intravitreal ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013; 156:652–60.

30). Cho JH, Ryoo NK, Cho KH, et al. Incidence rate of massive submacular hemorrhage and its risk factors in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2016; 169:79–88.

31). Pak KY, Park SW, Byon IS, Lee JE. Treat-and-extend regimen using rainbizumab for polypoidal choroidal vasculopathy: one-year results. Retina. 2017; 37:561–7.
Go to : 
 | Figure 1.A representative case of polypoidal choroidal vasculopathy treated with intravitreal aflibercept monotherapy. At diagnosis (A-C), the decimal best-corrected visual acuity (BCVA) was 0.4. Subretinal fluid was resolved after 3 monthly aflibercept injections (D). The patient was treated with 9 aflibercept injections during the 24 months follow-up period. At 24 months (E), The BCVA was measured as 0.5. (A) Fluorescein angiography. (B) Indocyanine green angiography. (C-E) Optical coherence tomography images. |
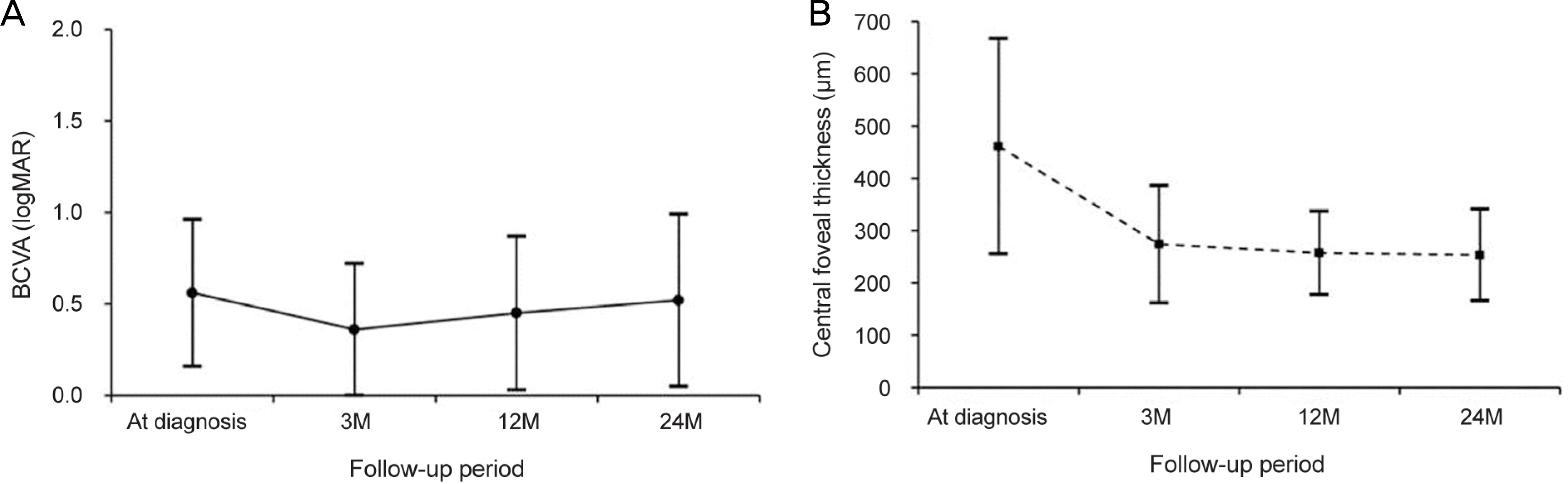 | Figure 2.Graphs showing functional and anatomical outcomes. Changes in the logarithm of the minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA) (A) and central foveal thickness (B) in the included patients (n = 46). M = months. |
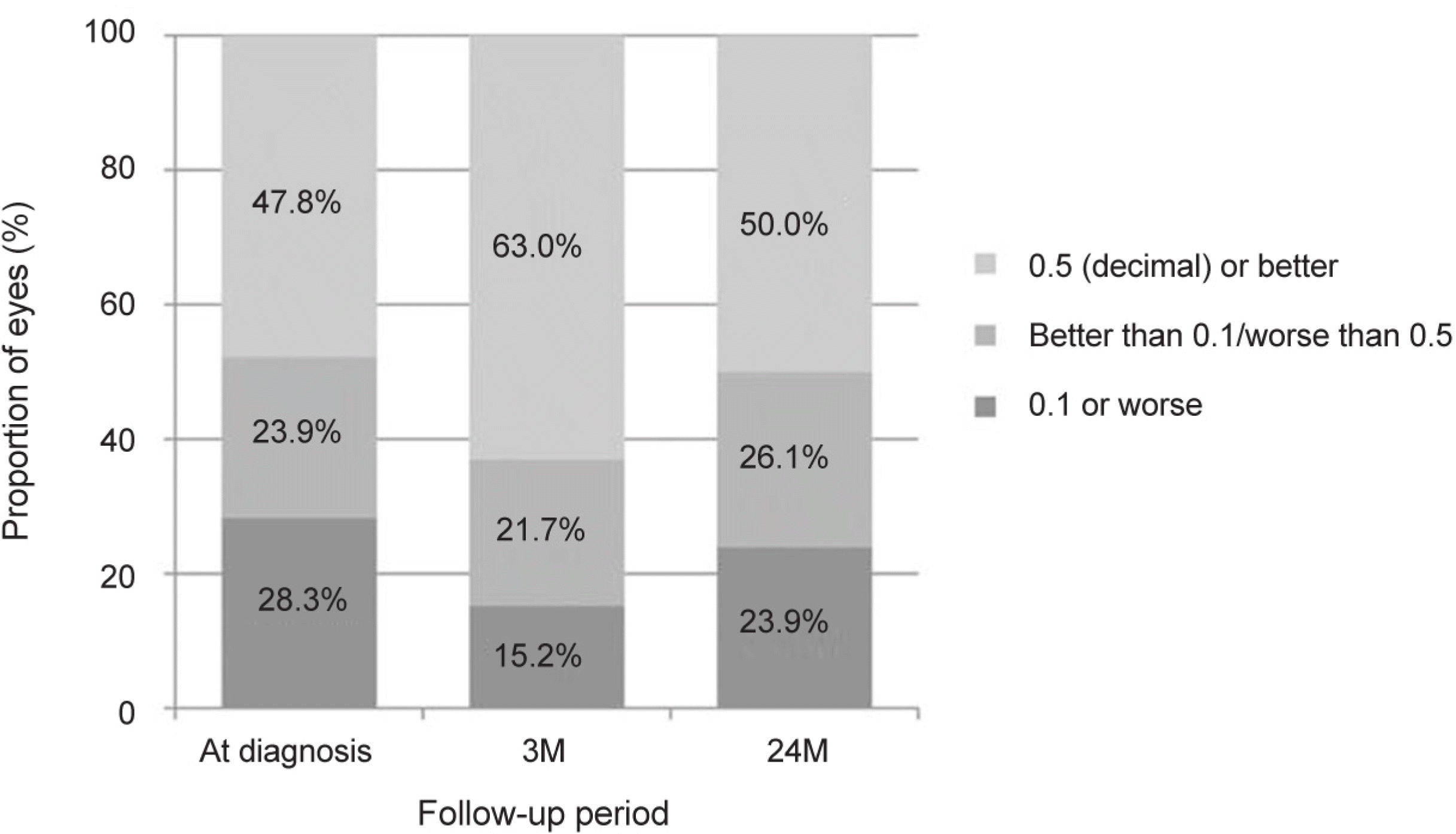 | Figure 3.Proportion of patients when stratified into best-corrected visual acuity (decimal) (n = 46). At 24 months, 76.1% show visual acuity better than 0.1. M = months. |
Table 1.
Characteristics of the included patients (n = 46)
Table 2.
Comparison of baseline morphologic features between patients, when divided into 2 groups, according to best-corrected visual acuity (decimal) at 24 months (n = 46)
| Characteristics | BCVA worse than 0.5 (n = 23) | BCVA 0.5 or better (n = 23) | p-value |
|---|---|---|---|
| Baseline central foveal thickness (n, %) | 1.000* | ||
| < 400 μm | 11 (47.8) | 11 (47.8) | |
| ≥ 400 μm | 12 (52.2) | 12 (52.2) | |
| Type of PCV (n, %) | 0.345* | ||
| Type 1 | 9 (39.1) | 6 (26.1) | |
| Type 2 | 14 (60.9) | 17 (73.9) | |
| Location of the polypoidal lesion (n, %) | 0.016* | ||
| Subfoveal/Juxtafoveal | 18 (78.3) | 10 (43.5) | |
| Extrafoveal | 5 (21.7) | 13 (56.5) | |
| Largest polyp diameter (n, %) | 0.555* | ||
| < 200 μm | 11 (47.8) | 13 (56.5) | |
| ≥ 200 μm | 12 (52.2) | 10 (43.5) | |
| Greatest linear dimension (n, %) | 0.767* | ||
| <2,500 μm | 12 (52.2) | 13 (56.5) | |
| ≥ 2,500 μm | 11 (47.8) | 10 (43.5) | |
| Fovea-involving PED (n, %) | 0.555* | ||
| Presence | 12 (52.2) | 10 (43.5) | |
| Absence | 11 (47.8) | 13 (56.5) |
Table 3.
Association between baseline morphologic features and best-corrected visual acuity at 24 months (n = 46)
| Morphologic features | p-value* |
|---|---|
| Baseline central foveal thickness | 0.772 |
| Type of PCV | 0.331 |
| Location of the polypoidal lesion | 0.020 |
| Largest polyp diameter | 0.516 |
| Greatest linear dimension | 0.994 |
| Fovea-involving PED | 0.796 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download