Abstract
Purpose
To report the first case of endophthalmitis due to Streptococcus dysgalactiae after phacoemulsification and posterior chamber intraocular lens implantation in the Republic of Korea.
Case summary
A 65-year-old male was transferred because of endophthalmitis following cataract surgery. His initial visual acuity was light perception. Because inflammation of the anterior chamber and vitreous cavity progressed rapidly, we performed total pars planar vitrectomy and intraocular lens extraction in addition to administering intravitreal antibiotics and intravitreal dexamethasone injections. Streptococcus dysgalactiae was identified in samples cultured from the vitreous and anterior chamber fluid. Four days after surgery, we washed the anterior chamber and intravitreal antibiotics were again injected because of increased inflammation of the anterior chamber and vitreous. The patient was discharged 25 days after surgery but corneal neovascularization, contraction, edema, infiltration, and hypopyon remained. Visual acuity progressed to no light perception and there was shrinkage of the globe.
Figures and Tables
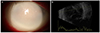
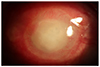
 | Figure 1Anterior segment photograph and ultrasonogram at the initial presentation. Anterior segment photography at first visit (A) Diffuse corneal edema and dense peripheral corneal infiltration and exudates in the anterior chamber. (B) Ultrasound B scan revealed the presence of dense vitreous infiltration. |
Notes
References
2. Jabbarvand M, Hashemian H, Khodaparast M, et al. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology. 2016; 123:295–301.
3. Lancefield RC. A serological differentiation of human and other groups of hemolytic streptococci. J Exp Med. 1933; 57:571–595.

4. Higgs TM, Neave FK, Bramley AJ. Differences in intramammary pathogenicity of four strains of Streptococcus dysgalactiae. J Med Microbiol. 1980; 13:393–399.

5. Sylvetsky N, Raveh D, Schlesinger Y, et al. Bacteremia due to beta-hemolytic Streptococcus group G: increasing incidence and clinical characteristics of patients. Am J Med. 2002; 112:622–626.

6. Ruppen C, Rasmussen M, Casanova C, Sendi P. A 10-year observational study of Streptococcus dysgalactiae bacteraemia in adults: frequent occurrence among female intravenous drug users. Swiss Med Wkly. 2017; 147:w14469.

7. Rantala S. Streptococcus dysgalactiae subsp. equisimilis bacteremia: an emerging infection. Eur J Clin Microbiol Infect Dis. 2014; 33:1303–1310.

8. Brandt CM, Spellerberg B. Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin Infect Dis. 2009; 49:766–772.
9. Takahashi T, Ubukata K, Watanabe H. Invasive infection caused by Streptococcus dysgalactiae subsp. equisimilis: characteristics of strains and clinical features. J Infect Chemother. 2011; 17:1–10.

10. Ramaswamy G, Ng A, Quinlan L, Tchertkoff V. Streptococcus equisimilis (group C) as a cause of ophthalmic infections. Am J Clin Pathol. 1983; 79:385–387.

11. Wickramasinghe N, Harris K. Bilateral endophthalmitis as a primary manifestation of Streptococcus dysgalactiae endocarditis and the role of 16S rDNA polymerase chain reaction in identification. Diagn Microbiol Infect Dis. 2010; 67:185–187.

12. Yong AS, Lau SY, Woo TH, et al. Streptococcus dysgalactiae endocarditis presenting as acute endophthalmitis. Infect Dis Rep. 2012; 4:e16.

13. Kaliamurthy J, Cuteri V, Jesudasen N, et al. Post-operative ocular infection due to Streptococcus dysgalactiae subspecies equisimilis. J Infect Dev Ctries. 2011; 5:742–744.

14. Miller JJ, Scott IU, Flynn HW Jr, et al. Acute-onset endophthalmitis after cataract surgery (2000-2004): incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol. 2005; 139:983–987.

15. Vaziri K, Schwartz SG, Kishor K, Flynn HW Jr. Endophthalmitis: state of the art. Clin Ophthalmol. 2015; 9:95–108.
16. Ravindran RD, Venkatesh R, Chang DF, et al. Incidence of post-cataract endophthalmitis at Aravind Eye Hospital: outcomes of more than 42,000 consecutive cases using standardized sterilization and prophylaxis protocols. J Cataract Refract Surg. 2009; 35:629–636.
17. Endophthalmitis Vitrectomy. Results of the Endophthalmitis Vitrectomy Study: A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol. 1995; 113:1479–1496.
18. Ciszewski M, Szewczyk EM. Potential factors enabling human body colonization by animal streptococcus dysgalactiae subsp. equisimilis Strains. Curr Microbiol. 2017; 74:650–654.

19. Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007; 447:279–283.

20. Schrieber L, Towers R, Muscatello G, Speare R. Transmission of Streptococcus dysgalactiae subsp. equisimilis between child and dog in an Aboriginal Australian community. Zoonoses Public Health. 2014; 61:145–148.
21. Silva LG, Genteluci GL, Corrêa de, et al. Group C Streptococcus dysgalactiae subsp. equisimilis in south-east Brazil: genetic diversity, resistance profile and the first report of human and equine isolates belonging to the same multilocus sequence typing lineage. J Med Microbiol. 2015; 64(Pt 5):551–558.

22. McNeilly CL, McMillan DJ. Horizontal gene transfer and recombination in Streptococcus dysgalactiae subsp. equisimilis. Front Microbiol. 2014; 5:676.

23. Broyles LN, Van Beneden C, Beall B, et al. Population-based study of invasive disease due to beta-hemolytic streptococci of groups other than A and B. Clin Infect Dis. 2009; 48:706–712.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download