Abstract
Purpose
To evaluate changes of the NLRP3 inflammasome complex and proinflammatory cytokine interleukin (IL)-1β in ocular tissues and cervical lymph nodes when the ocular surface was exposed to particulate matter.
Methods
Five-week-old Sprague Dawley male rats, each weighing 250–300 g, were used in this study. The rats were randomly divided into two groups involving the TiO2-exposed group (n = 12) and control group (n = 12). Rats in the TiO2-exposed group were exposed to airborne TiO2 microparticles in the exposure chamber twice daily for 2 hours for 5 days. The mean particulate matter (PM) 10 concentration in the exposure chamber was 331 ± 83 µg/m3. The score of corneal fluorescein staining for evaluating ocular surface damage and the size of cervical lymph nodes for evaluating the immune response in the lymphatic drainage pathway were measured. The expression of the nucleotide-binding domain leucine-rich repeats, pyrin domain-containing 3 (NLRP3) inflammasome complex (NLRP3, apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD) domain [ASC], and caspase-1) and IL-1β was quantitatively evaluated.
Results
In the TiO2-exposed group, the mean corneal stain score was significantly increased compared to that of the control group (p = 0.002), and the size of the cervical lymph nodes in the TiO2-exposed group was greater than that of the control group (p = 0.004). The expression of NLRP3 and ASC was significantly increased in the corneo-conjunctival tissue and cervical lymph nodes after TiO2 exposure (both, p = 0.037). Inactive precursor forms of caspase-1 and IL-1β were decreased, but active forms of caspase-1 and IL-1β were increased after TiO2 exposure (both, p = 0.037).
Conclusions
When the ocular surface was exposed to ambient particulate matter, the signal pathway of the NLRP3 inflammasome complex was activated, resulting in increased expression of the proinflammatory cytokine, IL-1β. The results suggested that the NLRP3 inflammasome complex was involved in the pathogenesis of ocular surface inflammation caused by exposure to particulate matter.
Figures and Tables
Figure 1
The mean corneal staining scores of the control and TiO2-exposed groups were compared. (A) Representative photographs of rat eyes after fluorescein staining in both groups. (B) A comparison of the mean corneal staining scores between the two groups. An asterisk (*) indicates a p-value < 0.05 by the Mann-Whitney U test (p = 0.002). Standard deviations are indicated by error bars.

Figure 2
The mean relative size of cervical lymph nodes in the control and TiO2-exposed groups were compared. (A) Representative photographs of cervical lymph nodes in both groups. (B) A comparison of cervical lymph node size between the two groups. An asterisk (*) indicates a p-value < 0.05 by the Mann-Whitney U test (n = 6, each group). Standard deviations are indicated by error bars. LN = lymph node.

Figure 3
The expression of nucleotide-binding domain leucine-rich repeats, pyrin domain-containing 3 (NLRP3) and apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD) domain (ASC) in the corneo-conjunctival tissues in the control and TiO2-exposed groups (n = 3, each group). (A) A representative example of a Western blot gel image and densitometry analysis for NLRP3. (B) A representative example of a Western blot gel image and densitometry analysis for ASC. β-Actin was used as an internal loading control for the Wetern blot. An asterisk (*) indicates a significant difference (p-value < 0.05) in the expression of NLRP3 and ASC in the TiO2-exposed group compared with the control group by the Mann-Whitney U test. Standard deviations are indicated by error bars.
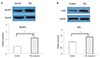
Figure 4
The expression of inactive precursor and active forms of caspase-1 and interleukin (IL)-1β in the corneo-conjunctival tissues in the control and TiO2-exposed groups (n = 3, each group). (A) A representative example of a Western blot gel image and densitometry analysis for pro-caspase-1 and caspase-1. (B) A representative example of a Western blot gel image and densitometry analysis for pro-IL-1β and IL-1β. β-Actin was used as an internal loading control for the Wetern blot. An asterisk (*) indicates a significant difference (p-value < 0.05) in the expression of inactive precursor and active forms of caspase-1 and IL-1β in the TiO2-exposed group compared with the control group by the Mann-Whitney U test. Standard deviations are indicated by error bars.

Figure 5
The expression of nucleotide-binding domain leucine-rich repeats, pyrin domain-containing 3 (NLRP3) and apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (CARD) domain (ASC) in the cervical lymph nodes in the control and TiO2-exposed groups (n = 3, each group). (A) A representative example of a Western blot gel image and densitometry analysis for NLRP3. (B) A representative example of a Western blot gel image and densitometry analysis for ASC. β-Actin was used as an internal loading control for the Wetern blot. An asterisk (*) indicates a significant difference (p-value < 0.05) in the expression of NLRP3 and ASC in the TiO2-exposed group compared with the control group by the Mann-Whitney U test. Standard deviations are indicated by error bars.
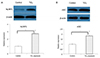
Figure 6
The expression of inactive precursor and active forms of caspase-1 and interleukin (IL)-1β in the cervical lymph nodes in the control and TiO2-exposed groups (n = 3, each group). (A) A representative example of a W estern blot gel image and densitometry analysis for pro-caspase-1 and caspase-1. (B) A representative example of a Western blot gel image and densitometry analysis for pro-IL-1β and IL-1β. β-Actin was used as an internal loading control for the Wetern blot. An asterisk (*) indicates a significant difference (p-value < 0.05) in the expression of inactive precursor and active forms of caspase-1 and IL-1β in the TiO2-exposed group compared with the control group by the Mann-Whitney U test. Standard deviations are indicated by error bars.

Notes
This study was presented as a narration at the 115th Annual Meeting of the Korea Ophthalmological Society 2016.
References
1. Brook RD, Rajagopalan S, Pope CA 3rd, et al. Particulate matterair pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010; 121:2331–2378.
2. Atkinson RW, Anderson HR, Sunyer J, et al. Acute effects of particulate air pollution on respiratory admissions: results from APHEA 2 project. Air Pollution and Health: a European Approach. Am J Respir Crit Care Med. 2001; 164(10 Pt 1):1860–1866.
3. Samoli E, Analitis A, Touloumi G, et al. Estimating the exposure-response relationships between particulate matter and mortality within the APHEA multicity project. Environ Health Perspect. 2005; 113:88–95.

4. Novaes P, Saldiva PH, Matsuda M, et al. The effects of chronic exposure to traffic derived air pollution on the ocular surface. Environ Res. 2010; 110:372–374.

5. Malerbi FK, Martins LC, Saldiva PH, Braga AL. Ambient levels of air pollution induce clinical worsening of blepharitis. Environ Res. 2012; 112:199–203.

6. Galor A, Kumar N, Feuer W, Lee DJ. Environmental factors affect the risk of dry eye syndrome in a United States veteran population. Ophthalmology. 2014; 121:972–973.

7. Eisenbarth SC, Flavell RA. Innate instruction of adaptive immunity revisited: the inflammasome. EMBO Mol Med. 2009; 1:92–98.

8. He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016; 41:1012–1021.

9. Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016; 13:148–159.

10. Zheng Q, Ren Y, Reinach PS, et al. Reactive oxygen species activated NLRP3 inflammasomes prime environment-induced murine dry eye. Exp Eye Res. 2014; 125:1–8.

11. Zheng Q, Ren Y, Reinach PS, et al. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp Eye Res. 2015; 134:133–140.

12. Warheit DB, Sayes CM, Reed KL, Swain KA. Health effects related to nanoparticle exposures: environmental, health and safety considerations for assessing hazards and risks. Pharmacol Ther. 2008; 120:35–42.

13. Sun Q, Tan D, Ze Y, et al. Pulmotoxicological effects caused by long-term titanium dioxide nanoparticles exposure in mice. J Hazard Mater. 2012; 235-236:47–53.

14. Chang X, Fu Y, Zhang Y, et al. Effects of Th1 and Th2 cells balance in pulmonary injury induced by nano titanium dioxide. Environ Toxicol Pharmacol. 2014; 37:275–283.

15. Hong J, Wang L, Zhao X, et al. Th2 factors may be involved in TiO2 NP-induced hepatic inflammation. J Agric Food Chem. 2014; 62:6871–6878.

16. Eom Y, Song JS, Lee DY, et al. Effect of titanium dioxide nanoparticle exposure on the ocular surface: an animal study. Ocul Surf. 2016; 14:224–232.

17. Eom Y, Song JS, Lee HK, et al. The effect of ambient titanium dioxide microparticle exposure to the ocular surface on the expression of inflammatory cytokines in the eye and cervical lymph nodes. Invest Ophthalmol Vis Sci. 2016; 57:6580–6590.

18. Han JY, Kang B, Eom Y, et al. Comparing the effects of particulate matter on the ocular surfaces of normal eyes and a dry eye rat model. Cornea. 2017; 36:605–610.

19. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:93–107.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download