Abstract
Purpose
To compare mini-mental state examination (MMSE) score between glaucoma group and normal control group and to evaluate the correlation between MMSE score and spectral domain-optical coherence tomography (SD-OCT) values in both groups.
Methods
This prospective study includes thirty glaucoma patients (eleven primary open angle glaucoma and nineteen normal tension glaucoma) and thirty normal controls. Retinal nerve fiber layer (RNFL) and Ganglion cell-inner plexiform layer (GC-IPL) thickness were measured with SD-OCT, and the average values of both eyes were used. The cognitive function was evaluated with MMSE by a single examiner.
Results
The mean MMSE scores of glaucoma group and normal group were 26.07 ± 2.95, and 27.00 ± 1.68 respectively (p = 0.137). MMSE score of less than 24 only showed in glaucoma group. MMSE score and RNFL thickness showed statistically no signifance in correlation (R2 = 0.236; p = 0.070), however, MMSE score and GC-IPL showed statistically significant correlation (R2 = 0.256; p = 0.048).
Go to : 
References
1. Resnikoff S, Pascolini D, Etya'ale D. . Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82:844–51.
2. Kim CS, Seong GJ, Lee NH. . Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology. 2011; 118:1024–30.
3. Ferri CP, Prince M, Brayne C. . Global prevalence of dementia: a Delphi consensus study. Lancet. 2005; 366:2112–7.

4. Mandas A, Mereu RM, Catte O. . Cognitive impairment and age-related vision disorders: their possible relationship and the evaluation of the use of aspirin and statins in a 65 years-and-over Sardinian population. Front Aging Neurosci. 2014; 6:309.

5. Lin IC, Wang YH, Wang TJ. . Glaucoma, Alzheimer’s disease, and Parkinson’s disease: an 8-year population-based follow-up study. PLoS One. 2014; 9:e108938.

6. Shiose Y, Kitazawa Y, Tsukahara S. . Epidemiology of glauco-ma in Japan–a nationwide glaucoma survey. Jpn J Ophthalmol. 1991; 35:133–55.
7. Henderson VW. The epidemiology of estrogen replacement ther-apy and Alzheimer’s disease. Neurology. 1997; 48((5 Suppl 7)):S27–35.

9. McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA. . Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest Ophthalmol Vis Sci. 2002; 43:1077–87.
10. Bayer AU, Ferrari F, Erb C. High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur Neurol. 2002; 47:165–8.

11. Yochim BP, Mueller AE, Kane KD, Kahook MY. Prevalence of cognitive impairment, depression, and anxiety symptoms among older adults with glaucoma. J Glaucoma. 2012; 21:250–4.

12. Kessing LV, Lopez AG, andersen PK, Kessing SV. No increased risk of developing Alzheimer disease in patients with glaucoma. J Glaucoma. 2007; 16:47–51.

13. Tamura H, Kawakami H, Kanamoto T. . High frequency of open-angle glaucoma in Japanese patients with Alzheimer’s disease. J Neurol Sci. 2006; 246:79–83.

14. Jefferis JM, Taylor JP, Collerton J. . The association between diagnosed glaucoma and cataract and cognitive performance in very old people: cross-sectional findings from the newcastle 85+ study. Ophthalmic Epidemiol. 2013; 20:82–8.

15. Cumurcu T, Cumurcu BE, Celikel FC, Etikan I. Depression and anxiety in patients with pseudoexfoliative glaucoma. Gen Hosp Psychiatry. 2006; 28:509–15.

16. Hagerman KE, Taussig MJ, Coalter JD, Jay WM. Low-vision re-habilitation in patients with visual and cognitive impairment. Vis Impair Res. 2007; 9:19–22.

17. Shen Y, Shi Z, Jia R. . The attenuation of retinal nerve fiber layer thickness and cognitive deterioration. Front Cell Neurosci. 2013; 7:142.

18. Oktem EO, Derle E, Kibaroglu S. . The relationship between the degree of cognitive impairment and retinal nerve fiber layer thickness. Neurol Sci. 2015; 36:1141–6.

Go to : 
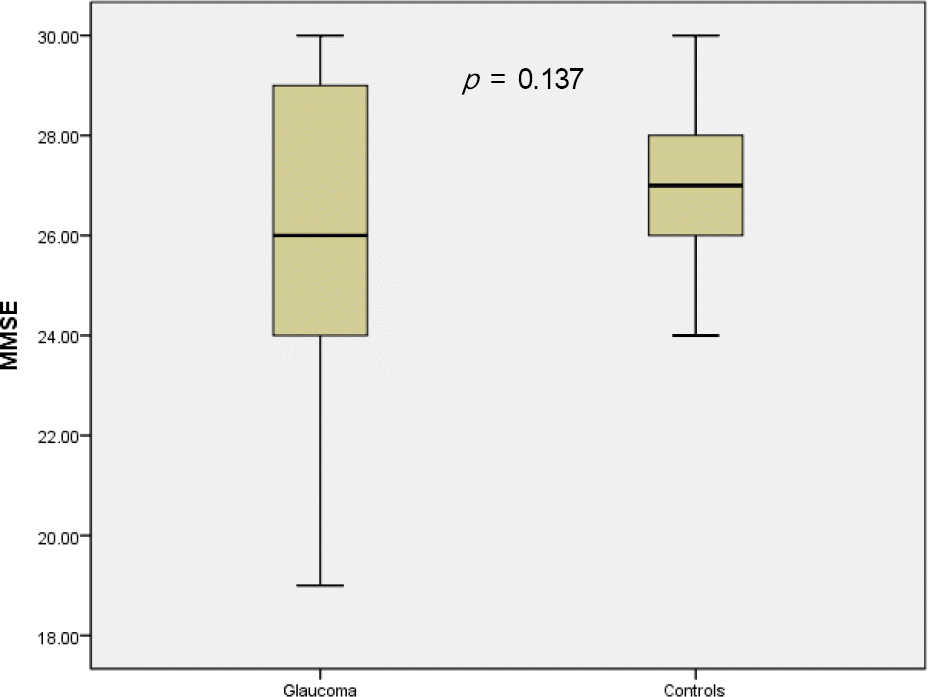 | Figure 1.Box plot graph of mini-mental state examination (MMSE) scores in glaucoma and control groups. The mean score of MMSE was 26.07 ± 2.95 in glaucoma group, and 27.00 ± 1.68 in normal control group, respectively. Glaucoma group showed low MMSE score than normal con-trol group, but it was not statistically significant. |
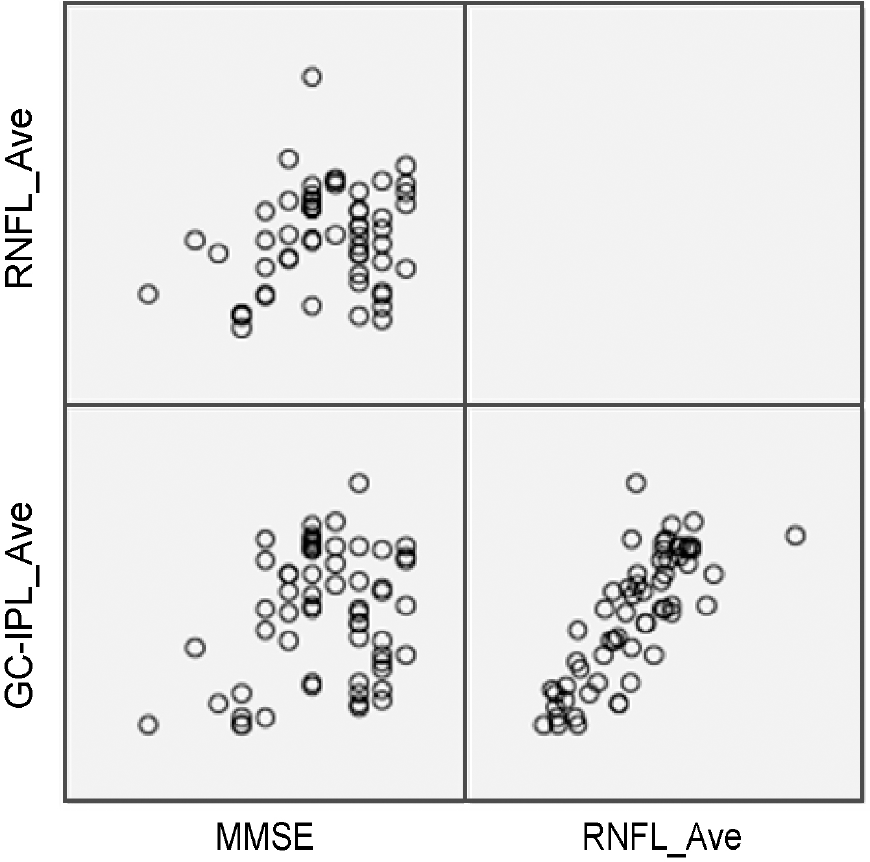 | Figure 2.Scatter plot of mini-mental state examination (MMSE) score and spectral domain– optical coherence tomography (SD-OCT) parameters. Scatter plot shows weak correlation be-tween MMSE score and SD-OCT parameters. RNFL = retinal nerve fiber layer; GC-IPL = ganglion cell-inner plexiform layer. |
Table 1.
Demographic and clinical characteristics of glaucoma and control subjects
| POAG (n = 11) | NTG (n = 19) | Control (n = 30) | p-value | Glaucoma (n = 30) | Control (n = 30) | p-value | |
|---|---|---|---|---|---|---|---|
| Age (years) | 68.63 ± 10.45 | 67.58 ± 9.89 | 66.10 ± 9.78 | 0.739* | 67.97 ± 9.93 | 66.10 ± 9.78 | 0.466* |
| Sex | |||||||
| Male | 8 (72.7) | 12 (63.2) | 11 (36.7) | 0.036† | 20 (66.7) | 11 (36.7) | 0.038† |
| Female | 3 (27.3) | 7 (36.8) | 19 (63.3) | 10 (33.3) | 19 (63.3) | ||
| Hypertension | |||||||
| Yes | 6 (54.5) | 11 (57.9) | 18 (60.0) | 0.115† | 17 (56.7) | 18 (60.0) | 1.000† |
| No | 5 (45.5) | 8 (42.1) | 12 (40.0) | 13 (43.3) | 12 (40.0) | ||
| Diabetic mellitus | |||||||
| Yes | 4 (36.4) | 5 (26.3) | 5 (16.7) | 0.091† | 9 (30.0) | 5 (16.7) | 0.360† |
| No | 7 (63.6) | 14 (73.7) | 25 (83.3) | 21 (70.0) | 25 (83.3) | ||
| BCVA | 0.20 ± 0.23 | 0.10 ± 0.11 | 0.09 ± 0.13 | 0.080* | 0.15 ± 0.22 | 0.09 ± 0.13 | 0.189* |
| Pseudophakia | |||||||
| Yes | 9 (81.8) | 6 (31.6) | 12 (40.0) | 0.110† | 15 (50.0) | 12 (40.0) | 0.604† |
| No | 2 (18.2) | 13 (68.4) | 18 (60.0) | 15 (50.0) | 18 (60.0) | ||
| MD (dB) | -19.17 ± 5.96 | -10.65 ± 8.66 | -13.78 ± 8.73 |
Table 2.
Comparison of the mean RNFL, GC-IPL, and MMSE between glaucoma and control groups
| POAG (n = 11) | NTG (n = 19) | Controls (n = 30) | p-value | Glaucoma (n = 30) | Control (n = 30) | p-value | |
|---|---|---|---|---|---|---|---|
| RNFL (μ m) | 68.05 ± 29.81 | 79.79 ± 18.10 | 97.22 ± 11.73 | <0.001* | 75.48 ± 23.30 | 97.22 ± 11.73 | <0.001* |
| GC-IPL (μ m) | 55.27 ± 8.11 | 60.82 ± 9.70 | 68.48 ± 5.80 | <0.001* | 58.78 ± 9.40 | 68.48 ± 5.80 | <0.001* |
| MMSE | 25.82 ± 3.46 | 26.21 ± 2.70 | 27.00 ± 1.68 | 0.305* | 26.07 ± 2.95 | 27.00 ± 1.68 | 0.137* |
Table 3.
The percentage of glaucoma patients in lower MMSE group and high MMSE group
| Glaucoma (n = 30) | Controls (n = 30) | p-value | ||
|---|---|---|---|---|
| POAG (n = 11) | NTG (n = 19) | |||
| Low MMSE | 3 (27.3) | 4 (21.1) | 0 (0) | 0.016* |
| High MMSE | 8 (72.7) | 15 (78.9) | 30 (100) | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download