Abstract
Purpose
To evaluate the effectiveness of optical coherence tomography angiography (OCTA) in investigating changes in the area of the foveal avascular zone (FAZ) in patients with diabetic retinopathy (DR) and normal subjects.
Methods
Eighty-five eyes of 50 DR patients and 50 eyes of 25 normal subjects were included. OCTA images of the FAZ were acquired using the split-spectrum amplitude decorrelation angiography algorithm of Optovue Avanti RTVue XR OCT. Patients were divided into three groups according to DR severity: mild-to-moderate non-proliferative diabetic retinopathy (NPDR) group, severe NPDR group, and proliferative DR group. The area of the FAZ was measured using built-in software and was compared between the patients and normal subjects and among the three groups.
Results
The area of the FAZ in patients with diabetic retinopathy (0.46 mm2) was significantly larger than that in normal subjects (0.30 mm2, p = 0.001). A significant difference was observed depending on DR severity: 0.40 mm2 in the mild-to-moderate NPDR group, 0.45 mm² in the severe NPDR group, and 0.53 mm2 in the PDR group (p = 0.03). Correlation between area of the foveal avascular zone and visual acuity showed a tendency toward reduction in visual acuity (p = 0.002).
Go to : 
References
1. Klein R, Klein BE, Moss SE. . The Wisconsin epidemiologic study of diabetic retinopathy: III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984; 102:527–32.
2. Arend O, Wolf S, Jung F. . Retinal microcirculation in patients with diabetes mellitus: dynamic and morphological analysis of perifoveal capillary network. Br J Ophthalmol. 1991; 75:514–8.

3. Aiello LP, Wong JS. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int Suppl. 2000; 77:S113–9.

4. Grant MB, Afzal A, Spoerri P. . The role of growth factors in the pathogenesis of diabetic retinopathy. Expert Opin Investig Drugs. 2004; 13:1275–93.

5. Arend O, Wolf S, Harris A, Reim M. The relationship of macular microcirculation to visual acuity in diabetic patients. Arch Ophthalmol. 1995; 113:610–4.

6. Sim DA, Keane PA, Zarranz-Ventura J. . The effects of macular ischemia on visual acuity in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013; 54:2353–60.

7. Kohner EM, Henkind P. Correlation of fluorescein angiogram and retinal digest in diabetic retinopathy. Am J Ophthalmol. 1970; 69:403–14.

8. Bresnick GH, De Venecia G, Myers FL. . Retinal ischemia in diabetic retinopathy. Arch Ophthalmol. 1975; 93:1300–10.

9. Jia Y, Bailey ST, Wilson DJ. . Quantitative optical coherence tomography angiography of choroidal neovascularization in age-re-lated macular degeneration. Ophthalmology. 2014; 121:1435–44.

10. Wilkinson CP, Ferris FL 3rd, Klein RE. . Proposed international clinical diabetic retinopathy and diabetic macular edema dis-ease severity scales. Ophthalmology. 2003; 110:1677–82.

11. Tick S, Rossant F, Ghorbel I. . Foveal shape and structure in a normal population. Invest Ophthalmol Vis Sci. 2011; 52:5105–10.

12. Snodderly DM, Weinhaus RS, Choi JC. Neural-vascular relation-ships in central retina of macaque monkeys (Macaca fascicularis). J Neurosci. 1992; 12:1169–93.

13. Paques M, Tadayoni R, Sercombe R. . Structural and hemody-namic analysis of the mouse retinal microcirculation. Invest Ophthalmol Vis Sci. 2003; 44:4960–7.

14. Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomog-raphy angiography. JAMA Ophthalmol. 2015; 133:45–50.

15. Shahlaee A, Pefkianaki M, Hsu J, Ho AC. Measurement of foveal avascular zone dimensions and its reliability in healthy eyes using optical coherence tomography angiography. Am J Ophthalmol. 2016; 161:50–5.e1.

16. Matsunaga DR, Yi JJ, De Koo LO. . Optical coherence tomog-raphy angiography of diabetic retinopathy in human subjects. Ophthalmic Surg Lasers Imaging Retina. 2015; 46:796–805.

17. Kashani AH, Lee SY, Moshfeghi A. . Optical coherence to-mography angiography of retinal venous occlusion. Retina. 2015; 35:2323–31.

18. Kim DY, Fingler J, Zawadzki RJ. . Noninvasive imaging of the foveal avascular zone with high-speed, phase-variance optical co-herence tomography. Invest Ophthalmol Vis Sci. 2012; 53:85–92.

19. Popovic Z, Knutsson P, Thaung J. . Noninvasive imaging of human foveal capillary network using dual conjugate adaptive optics. Invest Ophthalmol Vis Sci. 2011; 52:2649–55.
20. Novotny HR, Alvis DL. A method of photographing fluorescence in circulating blood in the human retina. Circulation. 1961; 24:82–6.

21. Mendis KR, Balaratnasingam C, Yu P. . Correlation of histo-logic and clinical images to determine the diagnostic value of fluo-rescein angiography for studying retinal capillary detail. Invest Ophthalmol Vis Sci. 2010; 51:5864–9.

22. Savastano MC, Lumbroso B, Rispoli M. In vivo characterization of retinal vascularization morphology using optical coherence to-mography angiography. Retina. 2015; 35:2196–203.

23. Ishibazawa A, Nagaoka T, Takahashi A. . Optical coherence tomography angiography in diabetic retinopathy: A Prospective Pilot Study. Am J Ophthalmol. 2015; 160:35–44.

24. Huang Y, Zhang Q, Thorell MR. . Swept-source OCT angiog-raphy of the retinal vasculature using intensity differentiation- based optical microangiography algorithms. Ophthalmic Surg Lasers Imaging Retina. 2014; 45:382–9.
25. Spaide RF, Klancnik JM Jr, Cooney MJ. Retinal vascular layers in macular telangiectasia type 2 imaged by optical coherence tomo-graphic angiography. JAMA Ophthalmol. 2015; 133:66–73.

26. Mansour AM, Schachat A, Bodiford G, Haymond R. Foveal avas-cular zone in diabetes mellitus. Retina. 1993; 13:125–8.

27. Bresnick GH, Condit R, Syrjala S. . Abnormalities of the fo-veal avascular zone in diabetic retinopathy. Arch Ophthalmol. 1984; 102:1286–93.

28. Conrath J, Giorgi R, Raccah D, Ridings B. Foveal avascular zone in diabetic retinopathy: quantitative vs qualitative assessment. Eye (Lond). 2005; 19:322–6.

29. Novais EA, Adhi M, Moult EM. . Choroidal neovascularization analyzed on ultrahigh-speed swept-source optical coherence to-mography angiography compared to spectral-domain optical coher-ence tomography angiography. Am J Ophthalmol. 2016; 164:80–8.

30. Scarinci F, Nesper P, Fawzi AA. Deep retinal capillary non-perfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol. 2016; 168:129–38.

31. Scarinci F, Jampl LM, Linsenmeier RA, Fawzi AA. Association of diabetic macular nonperfusion with outer retinal disruption on op-tical coherence tomography. JAMA Ophthalmol. 2015; 133:1036–44.

32. Yi J, Liu W, Chen S. . Visible light optical coherence tomog-raphy measures retinal oxygen metabolic response to systemic oxygenation. Light Sci Appl 2015;4. pii: e334. Epub 2015 Sep 25.

33. Samara WA, Say EA, Khoo CT. . Correlation of foveal avas-cular zone size with foveal morphology in normal eyes using opti-cal coherence tomography angiography. Retina. 2015; 35:2188–95.

34. Gadde SG, Anegondi N, Bhanushali D. . Quantification of ves-sel density in retinal optical coherence tomography angiography images using local fractal dimension vessel density in OCTA images. Invest Ophthalmol Vis Sci. 2016; 57:246–52.
35. Mammo Z, Balaratnasingam C, Yu P. . Quantitative noninvasive angiography of the fovea centralis using speckle variance optical co-herence tomography. Invest Ophthalmol Vis Sci. 2015; 56:5074–86.

36. Bonnin S, Mane V, Couturier A. . New insight into the macular deep vascular plexus imaged by optical coherence tomography angiography. Retina. 2015; 35:2347–52.

37. Parodi MB, Visintin F, Della Rupe P, Ravalico G. Foveal avascular zone in macular branch retinal vein occlusion. Int Ophthalmol. 1995; 19:25–8.

38. Balaratnasingam C, Inoue M, Ahn S. . Visual acuity is corre-lated with the area of the foveal avascular zone in diabetic retinop-athy and retinal vein occlusion. Ophthalmol. 2016; 123:2352–67.

39. Distler C, Weigel H, Hoffmann KP. Glia cells of the monkey retina. I. Astrocytes. J Comp Neurol. 1993; 333:134–47.
40. Gariano RF, Sage EH, Kaplan HJ, Hendrickson AE. Development of astrocytes and their relation to blood vessels in fetal monkey retina. Invest Ophthalmol Vis Sci. 1996; 37:2367–75.
Go to : 
 | Figure 1.Foveal avascular zone (FAZ) measured by optical coherence tomography angiography. Optical coherence tomog-raphy angiography clearly shows the area of the FAZ of the su-perficial retinal capillary plexus around the fovea. The margin of the FAZ was automatically demarcated and the area was measured by the built-in software. |
 | Figure 2.Optical coherence tomography angiography images of normal individuals and patients with diabetic retinopathy. (A) The foveal avascular zone (FAZ) in healthy individuals was round in shape and had regular borders (arrow). (B) The FAZ in patients with diabetic retinopathy had irregular borders (arrow). The pathologic capillary nonperfusion area was also frequently found outside the FAZ (arrowhead). The area of the FAZ in patients with diabetic retinopathy (B) was significantly larger than that in normal subjects. |
 | Figure 3.Comparison of the size of the foveal avascular zone (FAZ) according to diabetic retinopathy severity. Representative pho-tographs of the mild-to-moderate non-proliferative diabetic retinopathy (NPDR) (A), severe NPDR (B) and proliferative diabetic retinopathy (C) groups. A significant difference in the size of the FAZ was seen among the three groups. |
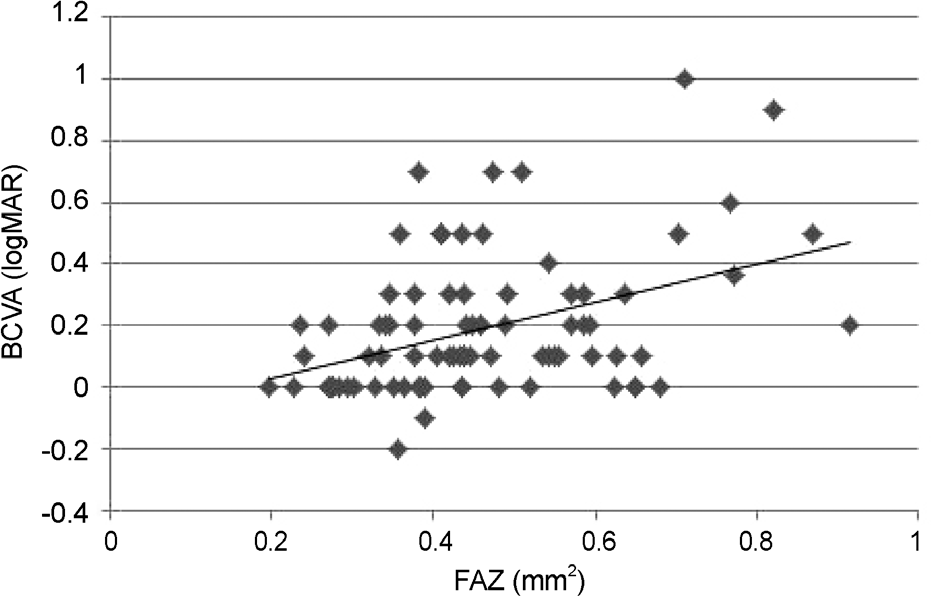 | Figure 4.Correlation between the foveal avascular zone and visual acuity. It showed a tendency towards reduction in visual acuity (Pearson correlation coefficient = 0.392, p = 0.002). BCVA = best corrected visual acuity; FAZ = foveal avascular zone; logMAR = logarithm of minimum angle of resolution. |
Table 1.
Comparison of the mean area of the foveal avascular zone between patients with diabetic retinopathy and normal subjects
| Normal subjects (n= 50 eyes) | Eyes with DR (n= 85 eyes) | p-value* | |
|---|---|---|---|
| Age (years) | 33.4 ± 9.8 | 57.2 ± 12.4 | |
| Sex (male:female) | 5:20 | 37:13 | |
| Area of FAZ (mm2) | 0.30 ± 0.10 | 0.46 ± 0.16 | 0.001* |
Table 2.
Comparison of the foveal avascular zone among diabetic retinopathy patients
| Mild-moderate NPDR (n= 19 eyes) | Severe NPDR (n= 43 eyes) | PDR (n= 15 eyes) | p-value* | |
|---|---|---|---|---|
| Age (years) | 55.3 ± 11.2 | 58.7 ± 12.9 | 57.3 ± 11.7 | |
| Sex (male:female) | 11:8 | 32:11 | 9:6 | |
| Area of FAZ (mm2) | 0.40 ± 0.12 | 0.45 ± 0.16 | 0.53 ± 0.14 | 0.03* |
Table 3.
Comparison of the mild-moderate NPDR group, severe NPDR group and PDR group
| Mild-moderate NPDR vs. Severe NPDR | Severe NPDR vs. PDR | Mild-moderate NPDR vs. PDR | |
|---|---|---|---|
| p-value* | 0.030 | 0.046 | 0.001 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download