Abstract
Purpose
The objective of this case report was to present tube erosion of Ahmed valve implantation using a synthetic dura substitute (Neuro-Patch®, B. Braun, Boulogne, France).
Case summary
Tube erosion was caused by dissolution of the conjunctiva and partial-thickness scleral tunnel in 5 patients who received Ahmed valve implantation using a synthetic dura substitute for glaucoma treatment 2 to 4 months after the operation. Furthermore, the patients required re-operation for preventing secondary complications such as endophthalmitis.
Figures and Tables
Figure 1
Image of Neuro-patch® (B. Braun, Boulogne, France). 3 × 3 mm Neuro-patchy was placed on scleral flap and covered with conjunctival flap.

Figure 2
Images of tube erosions with scleral melting in Ahmed valve implantation using a synthetic dura substitute (case 4). (A) One month after the operation, synthetic dura substitute was shown through the thinning conjunctival in the left eye. (B) The conjunctival thinning was aggravated for 2 weeks. (C) 3 months after the operation, Ahmed valve tube was exposed with melting sclera and conjunctiva and synthetic dura substitute was difficult to be recognized from the original shape. (D) Ahmed valve revision was performed with collagen glycoaminoglycan substitute.
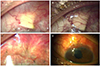
Figure 3
Four months after Ahmed valve implantation using a synthetic dura substitute in case 3. (A) Ahmed valve tube was exposed with conjunctival buttonhole in the left eye. (B) Ahmed valve body was exposed through the conjunctival buttonhole in right eye.

Table 1
The history of the patients with Ahmed valve implantation using a synthetic dura substitute

R = right; L = left; M = male; F = male; DM = diabetic mellitus; PDR = proliferative diabetic retinopathy; NVG = neovascular glaucoma; POAG = primary open-angle glaucoma; CRF = chronic renal failure; CAOD = coronary artery occlusive disease; OP = operation; HBV = hepatitis B-viral; IOL = intraocular lens.
References
1. Lim K, Allan BD, Lloyd AW, et al. Glaucoma drainage devices; past, present, and future. Br J Ophthalmol. 1998; 82:1083–1089.
2. Rosenberg LF, Krupin T. Implants in glaucoma surgery. The Glaucomas. 1996; 3:1783–1807.
3. Smith MF, Doyle JW, Ticrney JW Jr. A comparison of glaucoma drainage implant tube coverage. J Glaucoma. 2002; 11:143–147.
4. Rosentreter A, Mellein AC, Konen WW, Dietlein TS. Capsule excision and Ologen implantation for revision after glaucoma drainage device surgery. Graefes Arch Clin Exp Ophthalmol. 2010; 248:1319–1324.
5. Malliti M, Page P, Gury C, et al. Comparison of deep wound infection rates using a synthetic dural substitute (neuro-patch) or pericranium graft for dural closure: a clinical review of 1 year. Neurosurgery. 2004; 54:599–603. discussion 603-4.
6. Dubey S, Sharma V, Agrawal A, et al. Safety and efficacy of Ahmed glaucoma valve implantation in refractory glaucomas in Northern Indian eyes. Saudi J Ophthalmol. 2015; 29:103–108.
7. Kim SW, Kim YH, Yun IS, Ahn JH. Surgical treatment for tube erosion after Ahmed valve implantation. J Korean Ophthalmol Soc. 2016; 57:453–460.
8. Huang MC, Netland PA, Coleman AL, et al. Intermediate-term clinical experience with the Ahmed Glaucoma Valve implant. Am J Ophthalmol. 1999; 127:27–33.
9. Wilson MR, Mendis U, Paliwal A, Haynatzka V. Long-term follow-up of primary glaucoma surgery with Ahmed glaucoma valve implant versus trabeculectomy. Am J Ophthalmol. 2003; 136:464–470.
10. Heuer DK, Budenz D, Coleman A. Aqueous shunt tube erosion. J Glaucoma. 2001; 10:493–496.
11. Francis BA, DiLoreto DA, Chong LP, Rao N. Late-onset bacterial endophthalmitis following glaucoma drainage implantation. Ophthalmic Surg Lasers Imaging. 2003; 34:128–130.
12. Melamed S, Fiore PM. Molteno implant surgery in refractory glaucoma. Surv Ophthalmol. 1990; 34:441–448.
13. Trubnik V, Zangalli C, Moster MR, et al. Evaluation of risk factors for glaucoma drainage device-related erosions: a retrospective case-control study. J Glaucoma. 2015; 24:498–502.
14. Rosentreter A, Schild AM, Dinslage S, Dietlein TS. Biodegradable implant for tissue repair after glaucoma drainage device surgery. J Glaucoma. 2012; 21:76–78.
15. Gudmundsson G, Søgaard I. Complications to the use of vicryl-collagen dural substitute. Acta Neurochir (Wien). 1995; 132:145–147.
16. Raul JS, Godard J, Arbez-Gindre F, Czorny A. Use of polyester urethane (Neuro-Patch) as a dural substitute. Prospective study of 70 cases. Neurochirurgie. 2003; 49(2-3 Pt 1):83–89.
17. Cardwell RD, Kluge JA, Thayer PS, et al. Static and cyclic mechanical loading of mesenchymal stem cells on elastomeric, electrospun polyurethane meshes. J Biomech Eng. 2015; 137:DOI: 10.1115/1.4030404. Epub 2015 Jun 3.
18. Zanetta M, Quirici N, Demarosi F, et al. Ability of polyurethane foams to support cell proliferation and the differentiation of MSCs into osteoblasts. Acta Biomater. 2009; 5:1126–1136.
19. Huddleston SM, Feldman RM, Budenz DL, et al. Aqueous shunt exposure: a retrospective review of repair outcome. J Glaucoma. 2013; 22:433–438.
20. El Majdoub F, Löhr M, Maarouf M, et al. Transmigration of fibrino-purulent inflammation and malignant cells into an artificial dura substitute (Neuro-Patch): report of two cases. Acta Neurochir (Wien). 2009; 151:833–835.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download