Abstract
Purpose
To determine the prognostic factors associated with surgical time of endonasal dacryocystorhinostomy (DCR).
Methods
From April 2009 to June 2014, 66 eyes of 66 patients who underwent endonasal DCR for 5-year periods were retro-spectively evaluated with regard to surgical time and several other factors. The factors were patient factors (age, sex), category of diagnosis (inflammation and non-inflammation), and systemic factors (diabetes mellitus [DM], hypertension [HTN], anti-coagulant agents, sinusitis history). We divided the study period into three subperiods and compared their surgical time. The anatomical factor of thickness of the maxillary frontal process was evaluated by computed tomography (CT), as was the ex-istence of symptom recurrence after surgery and reoperation according to surgical time. A total of 66 cases (right: 31, left: 35) were included. Any case with concurrent surgery, abnormal structure of the nasal cavity, or bilateral DCR was excluded.
Results
Average surgical time was 49.95 minutes. Surgical time of endonasal DCR was short in inflammatory cases (p = 0.047), in the third surgical period (p = 0.001), and was correlated with thickness of the maxillary frontal process (p = 0.001). In addition, surgical time correlated with the existence of symptom recurrence after surgery and reoperation (p = 0.012).
Go to : 
References
1. Park JD, Kim YI, Shin SG. The factors related to surgical success rate of endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 1998; 12:2848–53.
2. Rice DH. Endoscopic intranasal dacryocystorhinostomy results in four patients. Arch Otolaryngol Head Neck Surg. 1990; 116:1061.

3. Javate RM, Campomanes BS Jr, Co ND. . The endoscope and the radiofrequency unit in DCR surgery. Ophthal Plast Reconstr Surg. 1995; 11:54–8.

4. Lee HC, Chung WS. Success rate of endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 1996; 37:211–8.
5. Boush GA, Lemke BN, Dortzbach RK. Results of endonasal la-ser-assisted dacryocystorhinostomy. Ophthalmology. 1994; 101:955–9.

6. Weidenbecher M, Hosemann W, Buhr W. Endoscopic endonasal dacryocystorhinostomy: results in 56 patients. Ann Otol Rhinol Laryngol. 1994; 103((5 Pt 1)):363–7.

7. Lee SH, Chung WS. Long term surgical efficacy of endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 2000; 41:307–13.
8. Kim C, Kacker A, Levine B, Lelli GJ. Lacrimal system endoscopy assisted endonasal dacryocystorhinostomy. Orbit. 2013; 32:156–60.

9. Yakopson VS, Flanagan JC, Ahn D, Luo BP. Dacryocystorhinostomy: History, evolution and future directions. Saudi J Ophthalmol. 2011; 25:37–49.

10. Tripathi A, Lesser TH, O’Donnell NP, White S. Local anaesthetic endonasal endoscopic laser dacryocystorhinostomy: analysis of patients’ acceptability and various factors affecting the success of this procedure. Eye (Lond). 2002; 16:146–9.

11. Kim JH, Shin JC. Clinical evaluation of endoscopic transnasal dacryocyocystorhinostomy. J Korean Ophthalmol Soc. 1997; 38:1706–11.
12. Zolli CL, Shannon GM. Dacryocystorhinostomy: a review of 119 cases. Ophthalmic Surg. 1982; 13:905–10.

13. McLachlan DL, Shannon GM, Flanagan JC. Results of dacryocys-torhinostomy: analysis of the reoperations. Ophthalmic Surg. 1980; 11:427–30.
14. Kong YT, Kim TI, Kong BW. A report of 131 cases of endoscopic laser lacrimal surgery. Ophthalmology. 1994; 101:1793–800.

15. Mannor GE, Millman AL. The prognostic value of preoperative da-cryocystography in endoscopic intranasal dacryocystorhinostomy. Am J Ophthalmol. 1992; 113:134–7.

16. Woo KI, Maeng HS, Kim YD. Characteristics of intranasal structures for endonasal dacryocy storhinostomy in Asians. Am J Ophthalmol. 2011; 152:491–8.e1.
17. Ha TS, Na KS, Chi NC. Effectiveness of washing nasolacrimal duct as an additional therapy after dacryocystorhinostomy. J Korean Ophthalmol Soc. 2000; 41:2308–12.
Go to : 
 | Figure 1.Measurement of the thickness of the maxillary fron-tal process by computed tomography scan. In axial view, we pointed the anterior lacrimal fossa (A) and maxillary-lacrimal suture line (B), and made parallel lines. At the center between two lines (mid-point of black line), thickness of frontal proc-ess was measured at medial side (double-headed arrow). |
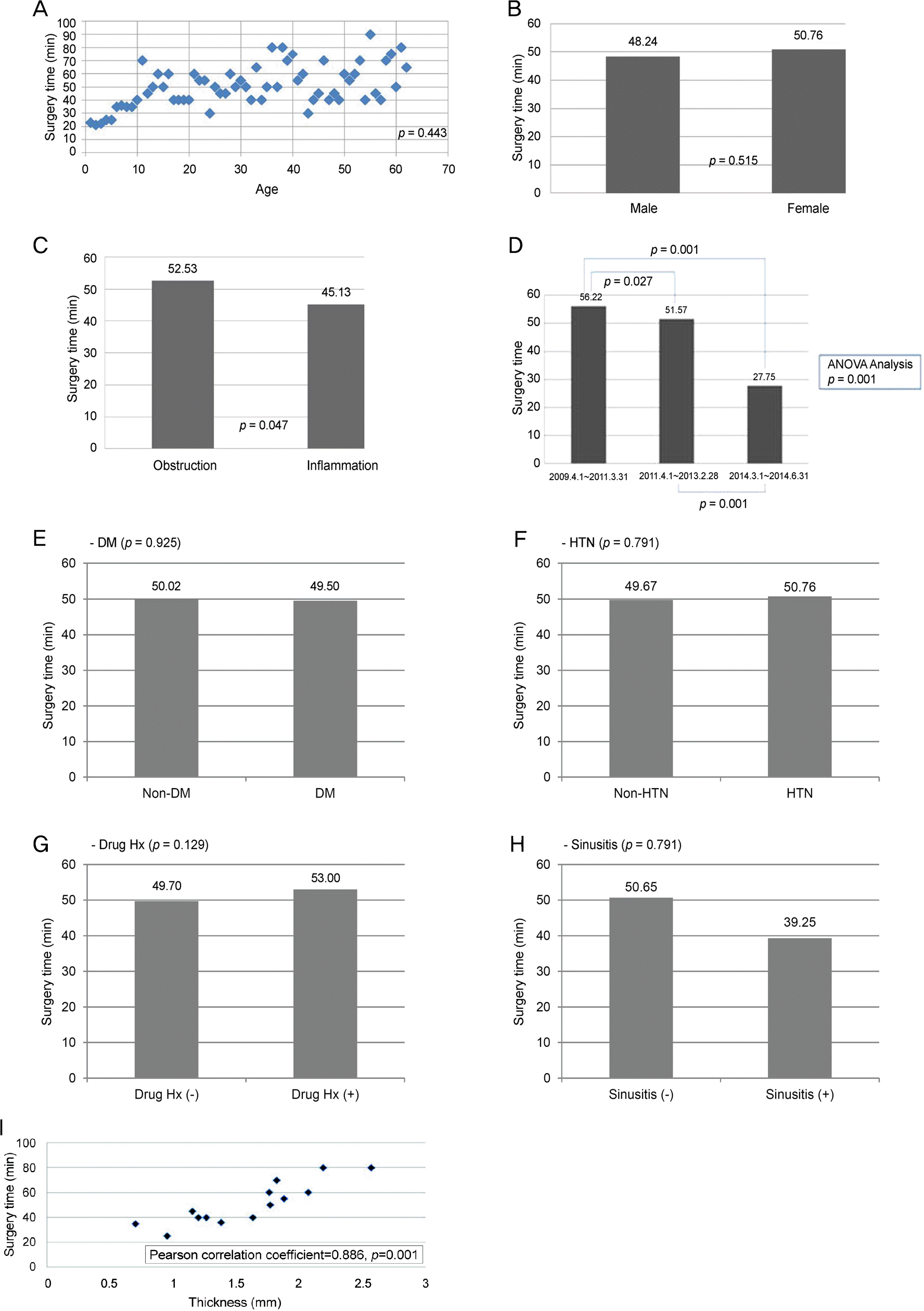 | Figure 2.Factors associated surgical time. Age (A), sex (B). Inflammation vs. non-inflammation (C). Period (D). Systemic factors (E-H). Thickness of maxillary frontal process (I). There were statisti-cally significant differences with disease category (Inflammation vs. non-inflammation; p = 0.047), period (p = 0.027. 0.001), thickness of maxillary frontal process (Pearson correlation coefficient = 0.886, p = 0.001). The average of thickness of frontal process of maxillary bone was 1.595 mm. But, there were no statistically significant differ-ences with age (p = 0.443), sex (p = 0.515), and systemic factors (DM; p = 0.925, HTN; p = 0.791, anticoagulant history; p = 0.129, sinusitis history; p = 0.791). ANOVA = analysis of variance; DM = diabetes mellitus; HTN = hypertension; Hx = history. |
Table 1.
Demographics of the patients
Table 2.
Factors associated surgical time
| Unstandardized coefficients | Standardized coefficients (Beta) | p-value* | |
|---|---|---|---|
| Age | 0.063 | 0.056 | 0.630 |
| Sex | 1.767 | 0.057 | 0.631 |
| Inflammation vs. Non-inflammation | -5.316 | -0.176 | 0.121 |
| Period | -10.548 | -0.475 | 0.000 |
| Thickness of maxillary frontal process | 9.145 | 0.578 | 0.000 |
Table 3.
Results of endonasal DCR related to surgical time
| Success (patients, %) | Failure (patients, %) | p-value* | |
|---|---|---|---|
| Group 1 (20-40 min) | 9 (19.6) | 2 (10.0) | 0.012 |
| Group 2 (40-60 min) | 25 (54.3) | 7 (35.0) | |
| Group 3 (60-80 min) | 12 (26.1) | 6 (30.0) | |
| Group 4 (80-100 min) | 0 (0.0) | 3 (15.0) | |
| Group 5 (over 100 min) | 0 (0.0) | 2 (10.0) | |
| Total | 46 (100.0) | 20 (100.0) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download