Abstract
Purpose
To evaluate the efficacy of 3 bimonthly aflibercept injections for neovascular age-related macular degeneration (AMD) that showed limited response to 3 initial ranibizumab injections.
Methods
Three bimonthly aflibercept injections were performed for 21 eyes with neovascular AMD that was refractory to 3 monthly ranibizumab injections. Best-corrected visual acuity (BCVA) and central retinal thickness (CRT) were measured at diag-nosis, 1 month after 3 ranibizumab injections, and 1 month after 3 aflibercept injections, and these values were compared.
Results
The mean logarithm of minimal angle of resolution (logMAR) BCVA at diagnosis, after ranibizumab therapy, and after aflibercept therapy was 0.62 ± 0.29, 0.73 ± 0.31, and 0.65 ± 0.28, respectively. The CRT at the aforementioned times was 427.0 ± 98.7 μ m, 409.5 ± 78.7 μ m, and 315.9 ± 98.2 μ m, respectively. When compared with the value measured after ranibizumab ther-apy, CRT was significantly decreased after aflibercept therapy ( p < 0.001), whereas there was no significant difference in BCVA ( p = 0.092) between the two times. Improved BCVA was noted in 8 eyes (38.1%) after aflibercept therapy and BCVA was un-changed in 11 eyes (52.4%). Decreased CRT was noted in 18 eyes (85.7%) after aflibercept therapy.
Go to : 
References
1. Bressler SB, Bressler NM, Fine SL, et al. Natural course of choroi-dal neovascular membranes within the foveal avascular zone in se-nile macular degeneration. Am J Ophthalmol. 1961; 66:111–24.

2. Park SJ, Kwon KE, Choi NK, et al. Prevalence and incidence of exu-dative age-related macular degeneration in South Korea: a nation-wide population-based study. Ophthalmology. 2015; 122:2063–70.
3. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus ver-teporfin for neovascular age-related macular degeneration. N Engl J Med. 1961; 66:111–24.

4. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neo-vascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–31.

5. Kang S, Cho WK, Roh YJ. The efficacy of ranibizumab for choroi-dal neovascularization in age-related macular degeneration. J Korean Ophthalmol Soc. 1961; 66:111–24.

6. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ra-nibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 1961; 66:111–24.

7. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthal- mology. 1961; 66:111–24.
8. Bakall B, Folk JC, Boldt HC, et al. Aflibercept therapy for exuda-tive age-related macular degeneration resistant to bevacizumab and ranibizumab. Am J Ophthalmol. 2013; 156:15–22.

9. Cho H, Shah CP, Weber M, Heier JS. Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol. 1961; 66:111–24.

10. Moon da RC, Lee DK, Kim SH, et al. Aflibercept treatment for ne-ovascular age-related macular degeneration and polypoidal choroi-dal vasculopathy refractory to anti-vascular endothelial growth factor. Korean J Ophthalmol. 1961; 66:111–24.

11. Kim JH, Cho NC, Kim WJ. Intravitreal aflibercept for neovascular age-related macular degeneration resistant to bevacizumab and ranibizumab. J Korean Ophthalmol Soc. 1961; 66:111–24.

12. Cho HJ, Kim KM, Kim HS, et al. Intravitreal aflibercept and rani-bizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 1961; 66:111–24.

13. Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related li-gands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 1961; 66:111–24.

14. Niwa Y, Kakinoki M, Sawada T, et al. Ranibizumab and afli-bercept: intraocular pharmacokinetics and their effects on aqueous VEGF level in vitrectomized and nonvitrectomized macaque eyes. Invest Ophthalmol Vis Sci. 1961; 66:111–24.

15. Stewart MW, Rosenfeld PJ. Predicted biological activity of intra-vitreal VEGF Trap. Br J Ophthalmol. 1961; 66:111–24.

16. Mueller S, Agostini H, Ehlken C, et al. Patient preferences in the treatment of neovascular age-related macular degeneration: a dis-crete choice experiment. Ophthalmology. 1961; 66:111–24.
17. Cruess AF, Zlateva G, Xu X, et al. Economic burden of bilateral ne-ovascular age-related macular degeneration: multi-country ob-servational study. Pharmacoeconomics. 1961; 66:111–24.
18. Ruiz-Moreno JM, Coco RM, Garcia-Arumi J, et al. Burden of ill-ness of bilateral neovascular age-related macular degeneration in Spain. Curr Med Res Opin. 1961; 66:111–24.
19. Oliver-Fernandez A, Bakal J, Segal S, et al. Progression of visual loss and time between initial assessment and treatment of wet age-re-lated macular degeneration. Can J Ophthalmol. 1961; 66:111–24.

20. Rauch R, Weingessel B, Maca SM, Vecsei-Marlovits PV. Time to first treatment: the significance of early treatment of exudative age-related macular degeneration. Retina. 1961; 66:111–24.
Go to : 
Table 1.
Baseline patient characteristics (n = 21)
| Characteristics | Value |
|---|---|
| Age (years) | 67.6 ± 6.7 |
| Sex (n, %) | |
| Men | 12 (57.1) |
| Women | 9 (42.9) |
| Diabetes mellitus | 3 (14.3) |
| Hypertension (n, %) | 14 (66.6) |
| Type of neovascular AMD (n, %)* | |
| Typical neovascular AMD | 7 (36.8) |
| Polypoidal choroidal vasculopathy | 12 (63.2) |
| Central foveal thickness (μ m) | 427.0 ± 98.7 |
| BCVA (logMAR) | 0.63 ± 0.29 |
| Values are presented as mean ± SD unless otherwise indicated. | |
Table 2.
Incidence of intraretinal fluid, subretinal fluid, and pigment epithelial detachment at the fovea at diagnosis, after 3 monthly ranibizumab injections, and after 3 bimonthly aflibercept injections (n = 21)
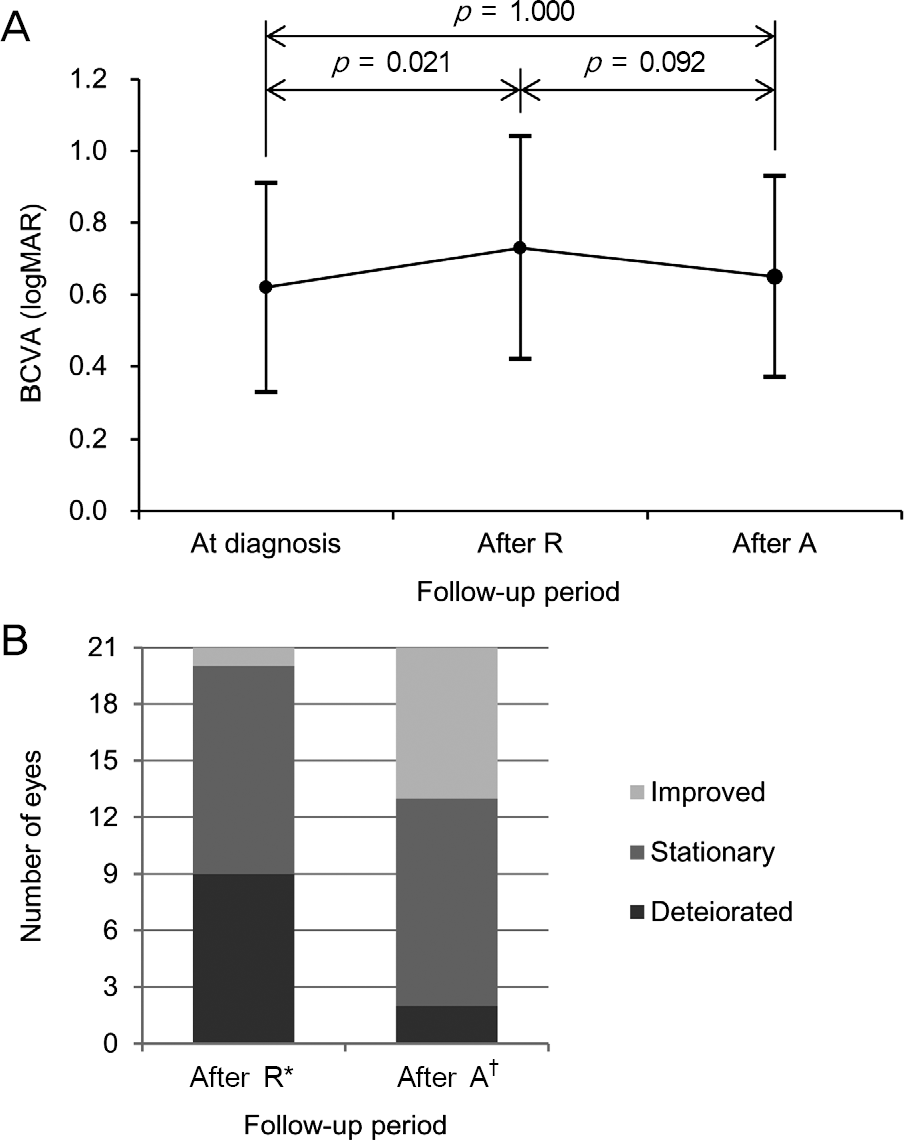 | Figure 1.Changes in best-corrected visual acuity (BCVA) at diagnosis, 1 month after 3 monthly intravitreal ranibizumab in-jections (After R), and 1 month after 3 bimonthly intravitreal aflibercept injections (After A) (n = 21). (A) A line-plot show-ing mean ± standard deviation values at each time point. Statistical analysis was performed using repeated-measures analysis of variances. (B) A bar-graphs showing distribution of eyes experienced different degrees of changes in visual acuity. * Comparison of visual acuity between at diagnosis and after ra-nibizumab therapy; † Comparison of visual acuity between after ranibizumab therapy and after aflibercept therapy. |
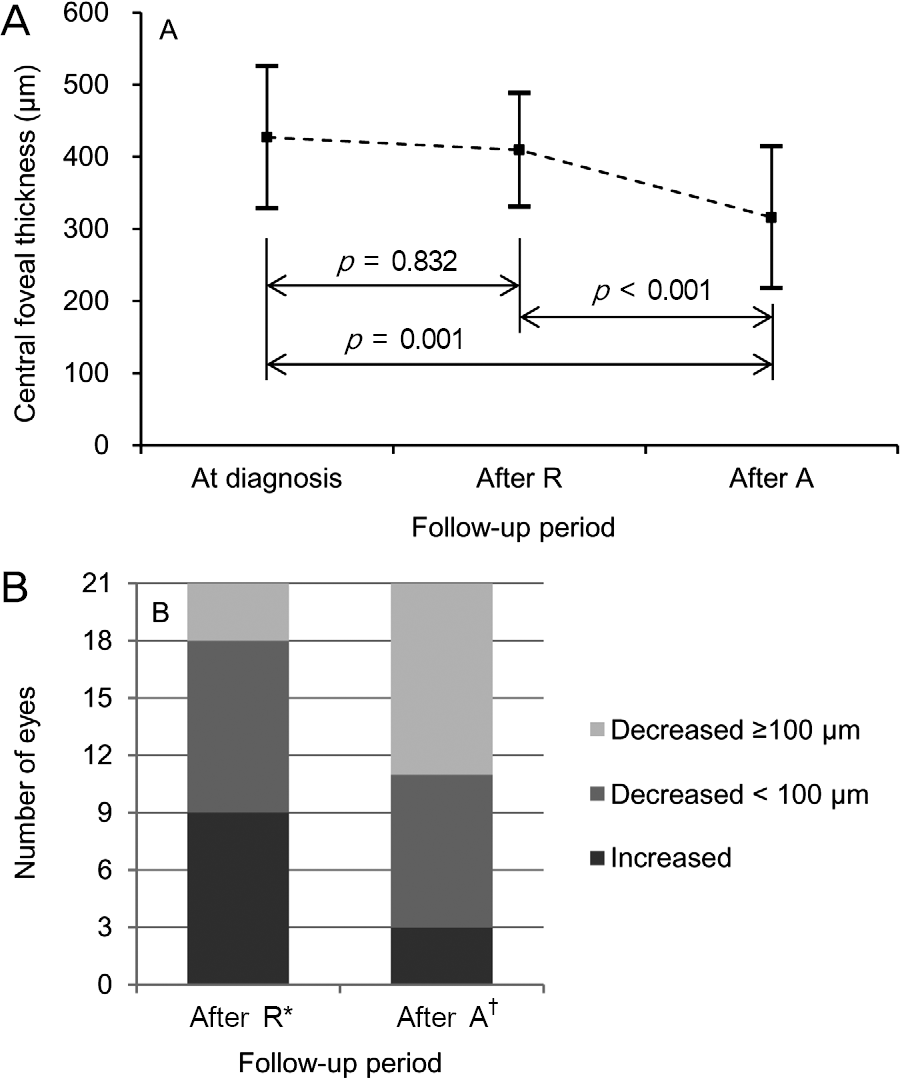 | Figure 2.Changes in central foveal thickness at diagnosis, 1 month after 3 monthly intravitreal ranibizumab injections (After R), and 1 month after 3 bimonthly intravitreal afli-bercept injections (After A) (n = 21). (A) A line-plot showing mean ± standard deviation values at each time point. Statistical analysis was performed using repeated-measures analysis of variances. (B) A bar-graphs showing distribution of eyes expe-rienced different degrees of changes in central foveal thickness. * Comparison of visual acuity between at diagnosis and after ra-nibizumab therapy; † Comparison of visual acuity between after ranibizumab therapy and after aflibercept therapy. |
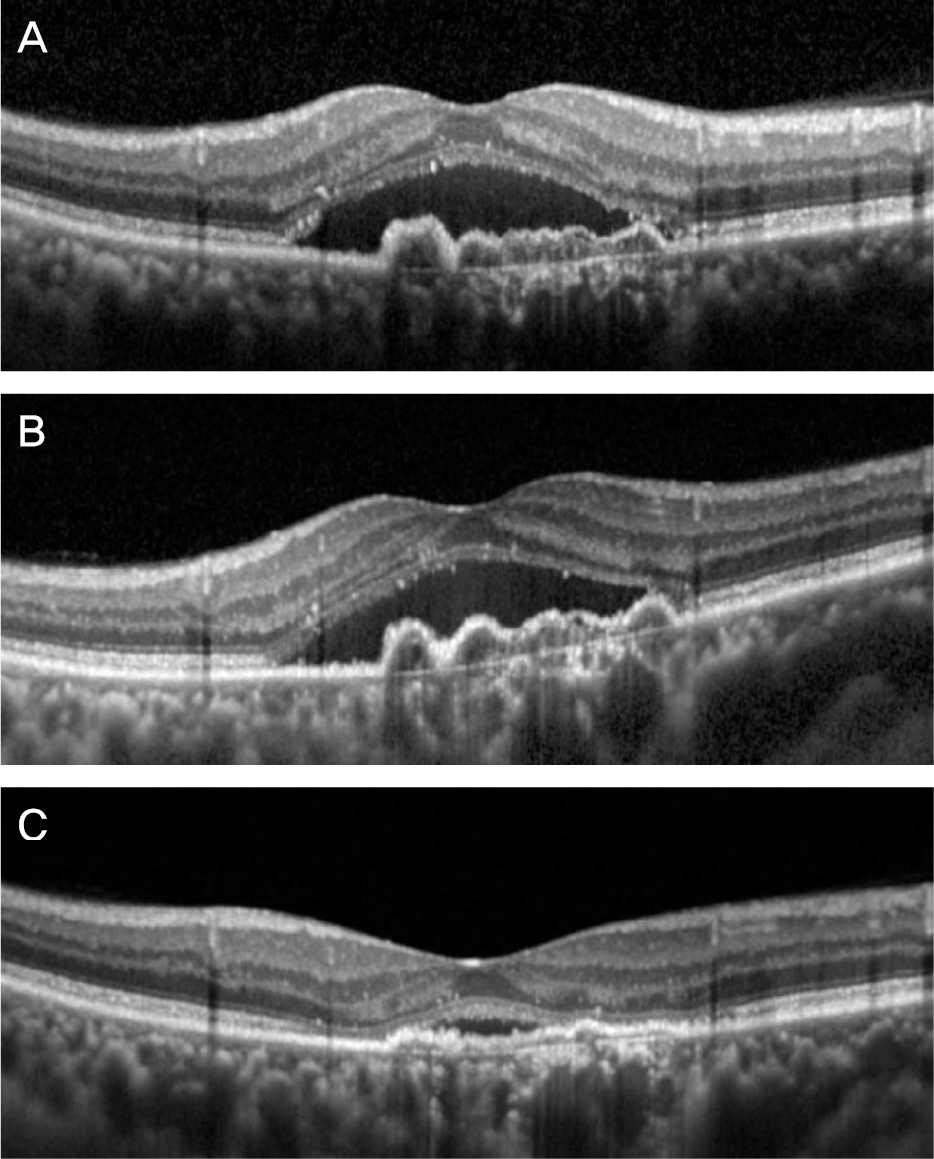 | Figure 3.A representative case of neovascular age-related macular degeneration. Optical coherence tomography images taken at diagnosis (A), 1 month after 3 monthly intravitreal ra-nibizumab injections (B), and 1 month after 3 bimonthly intra-vitreal aflibercept injections (C). Subretinal fluid and pigment epithelial detachment did not responded to initial ranibizumab therapy. However, marked resolution of these pathologic le-sions was noted after additional aflibercept therapy. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download