Abstract
Purpose
To evaluate the efficacy of endonasal revision using triamcinolone-soaked nasal packing in patients exhibiting recurrence of epiphora after endoscopic dacryocystorhinostomy.
Case summary
Four patients (4 eyes) who presented with the chief complaint of recurrence of epiphora after endoscopic dacryocystorhinostomy underwent endonasal revision under local anesthesia. On nasal endoscopy, granulation tissue and membranous tissue around the osseous foramen was removed during endonasal revision. According to the operator's judgement, the osseous foramen was additionally expanded. Following insertion of a silicone tube, triamcinolone-soaked nasal packing was used for intra-nasal packing. The silicone tube was removed after follow-up of more than 12 weeks. Immediately after removing the silicone tube, there was free passage of saline on lacrimal syringing as well as complete resolution of epiphora. At over 6 months of follow-up after tube removal, there was no recurrence of epiphora in any of the 4 patients.
Figures and Tables
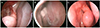
Figure 1
Endoscopic view of case 1. (A) Granulomatous obstruction of bony opening at 2 months after endonasal dacryocystorhinostomy (arrow). (B) After 2 weeks of removal of granulomatous tissue and silicone tube insertion using triamcinolone-soaked synthetic sponge (Nasopore®). (C) Patent bony opening at 6 months after tube removal.
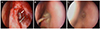
Figure 2
Endoscopic view of case 4. (A) Membranous obstruction of bony opening at 3 months after endonasal dacryocystorhinostomy (arrow). (B) After removal of membranous tissue, silicone tube insertion and triamcinolone-soaked synthetic sponge (Nasopore®) was used for the intra-nasal packing. (C) Patent bony opening at 6 months after tube removal.
References
1. Ben Simon GJ, Joseph J, Lee S, et al. External versus endoscopic dacryocystorhinostomy for acquired nasolacrimal duct obstruction in a tertiary referral center. Ophthalmology. 2005; 112:1463–1468.
2. Lee TS, Shin HH, Hwang SJ, Baek SH. The results of revisional surgery for the failed endonasal DCR. J Korean Ophthalmol Soc. 2007; 48:186–192.
3. Côté DW, Wright ED. Triamcinolone-impregnated nasal dressing following endoscopic sinus surgery: a randomized, double-blind, placebo-controlled study. Laryngoscope. 2010; 120:1269–1273.
4. Ali MJ, Wormald PJ, Psaltis AJ. The dacryocystorhinostomy ostium granulomas: classification, indications for treatment, management modalities and outcomes. Orbit. 2015; 34:146–151.
5. Camara JG, Bengzon AU, Henson RD. The safety and efficacy of mitomycin C in endonasal endoscopic laser-assisted dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2000; 16:114–118.
6. Ragab SM, Elsherif HS, Shehata EM, et al. Mitomycin C-enhanced revision endoscopic dacryocystorhinostomy: a prospective randomized controlled trial. Otolaryngol Head Neck Surg. 2012; 147:937–942.
7. Roozitalab MH, Amirahmadi M, Namazi MR. Results of the application of intraoperative mitomycin C in dacryocystorhinostomy. Eur J Ophthalmol. 2004; 14:461–463.
8. Leibovitch I, Prabhakaran VC, Davis G, Selva D. Intraorbital injection of triamcinolone acetonide in patients with idiopathic orbital inflammation. Arch Ophthalmol. 2007; 125:1647–1651.
9. Ebner R, Devoto MH, Weil D, et al. Treatment of thyroid associated ophthalmopathy with periocular injections of triamcinolone. Br J Ophthalmol. 2004; 88:1380–1386.
10. Li EY, Cheng AC, Wong AC, et al. Safety and efficacy of adjunctive intranasal mitomycin C and triamcinolone in endonasal endoscopic dacryocystorhinostomy. Int Ophthalmol. 2016; 36:105–110.
11. Zeldovich A, Ghabrial R. Revision endoscopic dacryocystorhinostomy with betamethasone injection under assisted local anaesthetic. Orbit. 2009; 28:328–331.
12. Ahn SM, Kim SS. Clinical application of polyether ester urethane in endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 2012; 53:743–748.
13. Baek JS, Jang SY, Park TS, et al. Clinical results of Nasopore(R) nasal packing on endonasal dacryocystorhinostomy. J Korean Ophthalmol Soc. 2013; 54:557–561.
14. Go Y, Park J, Kim K, Lee S. Comparison of nonlaser endoscopic endonasal revision surgery and diode laser transcanalicular revision surgery for failed dacryocystorhinostomy. J Craniofac Surg. 2015; 26:863–866.
15. Jeon HM, Ahn DS, Roh JH. Surgical outcomes of endonasal revision surgery for failed DCR according to number of silicone tubes. J Korean Ophthalmol Soc. 2015; 56:651–655.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download